INTRODUCTION
1. This is my judgment from the trial of this action concerned with three patents relating to microbial oils and their production. These oils can contain a high level of polyunsaturated fatty acids (“PUFAs”), such as an omega-3 PUFA called docosahexaenoic acid (“DHA”) which traditionally has been sourced from fish oil, and which is an important component of infant formula.
2. The Claimants (collectively “DSM”) and the Defendants (collectively “Mara”) are competing producers of such oils (it being unnecessary for present purposes to distinguish as between the two Claimants and as between the two Defendants).
3. DSM asserts infringement of three patents (from three different families). In order of priority date, the patents are as follows (there is no challenge to priority).
i) EP 2 921 155 (“EP155”) entitled “Methods for producing high-quality lipids by enzymatic liberation from biomass” (priority date 3 May 2002). It relates to the use of a protease enzyme in the process to extract microbial oil from Schizochytrium microorganisms. It expired on 4 May 2023;
ii) EP 3 530 740 (“EP740”) entitled “Thraustochytrids, fatty acid compositions, and methods of making and uses thereof” (filing date 19 March 2009 and no earlier priority date). It provides a new microbial oil, which is a crude oil product derived from a Thraustochytrid microorganism, which has a high triglyceride fraction and a high percentage of DHA in that triglyceride fraction.
iii) EP 2 576 801 (“EP801”) entitled “Extraction of lipid from cells and products therefrom” (earliest priority date 01 June 2010). It relates to a microbial oil extraction process which solves the emulsion problem without the use of organic solvents.
4. DSM were keen to emphasise that all three patents have been considered by the EPO Opposition Division (“OD”):
i) EP155 was maintained as granted by the OD. Opposition proceedings were commenced in 2020 by a strawman entity, and following oral proceedings on 28 September 2022 the opposition was rejected. That decision was not appealed.
ii) EP740 was upheld in amended form by the OD. Opposition proceedings were commenced in 2022 by a strawman entity (another opponent unrelated to the parties to these proceedings initially also opposed EP 740 but withdrew its opposition at an early stage). The First and Second Defendants in these proceedings each filed interventions in March and April 2023 respectively, on the basis of the present proceedings. Following oral proceedings held on 23 November 2023 the OD found claim 1 as granted invalid for added matter, but upheld EP 740 amended in a form corresponding to claim 2 as granted. An appeal to the Technical Boards of Appeal is outstanding, with the appeal hearing date yet to be set.
iii) EP801 was upheld in amended form by the OD. Opposition proceedings were commenced in 2020 by a strawman entity. The First and Second Defendants in these proceedings each filed interventions in March and April 2023 respectively, on the basis of the present proceedings. Following the oral proceedings of 10 and 11 September 2024 the OD found claim 1 as granted invalid for added matter, but upheld EP 801 on the basis of Auxiliary Request 2 (being claim 1, with step c1 (salt addition) and one or more of steps c2 to c4 being required). At the trial the written reasons of the OD were yet to be provided.
5. Mara counterclaims for invalidity on a variety of grounds. Much of the prior art comes from DSM. The same inventors are named on both Bijl and Hendrik, and Kobzeff (prior art to EP801) is closely related to EP155. One of the experts, Mr Dueppen, exhibited comparisons to show the common text. DSM has applied to amend unconditionally both EP740 (an amendment opposed on several grounds) and EP801 (opposed on the basis that the amendment does not cure the invalidity).
6. The claims said to be independently valid and infringed are:
i) EP155: claims 1 and 5.
ii) EP740: claims 1B and 2B (these being claims from the claim set at Annex B of a second amendment application, which is the only claim set now relied on).
iii) EP801: claims 1A, 6A & 7A (these being claims from the claim set at Annex A of the amendment application). I note in passing that shortly before trial, DSM confirmed that it no longer seeks to introduce claim 2A.
7. Mara has carried out (and proposes to carry out) a variety of processes to produce two oils: a lower-DHA oil called Mara DHA, and a higher-DHA oil called Mara DHA Plus. These use different microbial strains and different process conditions.
8. Mara DHA is Mara’s standard DHA microbial oil product obtained from a type of Thraustochytrid microorganism designated as strain ONC-T18 (or “T18”). Mara DHA Plus is Mara’s high DHA oil product (>55% DHA) obtained from a different Thraustochytrid designated as “G3”. There are a number of different versions of the processes in issue but the parties have agreed that infringement may be tried by reference to a limited number of exemplary processes.
9. In summary:
i) For EP155, the processes for production of both Mara DHA and Mara DHA Plus are said to infringe claim 1, but only the production of Mara DHA is now said to infringe claim 5. Subject to validity, infringement of claim 1 is admitted but infringement of claim 5 is denied. Claim 5 was relied upon as being independently valid for the purposes of sufficiency only (not obviousness), but in closing Mara indicated they did not pursue insufficiency of EP155.
ii) For EP740, which claims a high DHA oil with certain features, only Mara DHA Plus is alleged to infringe. The only infringement issue for determination is over the numerical limits in claims 1B and 2B, which affects which batches fall within those limits.
iii) For EP801, the processes for production of both Mara DHA and Mara DHA Plus are said to infringe, which is denied. After recent streamlining of Mara’s case, the non-infringement points relate to pH (it being a requirement of the claim that the pH is raised to 8 or above).
10. Finally, and for reasons which will become apparent, it is necessary to introduce the concepts of upstream and downstream processing. Upstream processing covers strain selection, media development and process design to enable the production of a lipid rich biomass at a commercial scale and downstream processing covers cell inactivation, extracting the oil from the cells and recovering the crude oil, and refining the oil.
11. DSM called two expert witnesses. First, Mr Daniel Dueppen. He gave evidence from the perspective of the Skilled Bioprocessing Engineer, a person skilled in downstream processing. Mr Dueppen has 40 years of experience relating to the use of microorganisms to produce a range of substances with potential medical, human or animal health applications. He is currently Director of Bioprocess Development at HTL Biotechnology in New Jersey, working on fermentation and downstream processing. Mr Dueppen has a degree in Agronomy, which he completed in 1985. Up until 2000, he had roles in companies involved in producing products by fermentation. In 2000, Mr Dueppen joined OmegaTech Inc. as Production Manager where he worked on, and improved, the production methods of one of the first commercial DHA omega-3 oils produced from a microorganism. After OmegaTech was acquired by Martek in 2002, Mr Dueppen worked as RBD Production Manager, taking control of the refining, bleaching and deodorizing process. This included co-managing and designing RBD production as part of the construction of a major facility for microbial oil production. In 2004, as Third-Party Production Manager, he worked on qualifying omega-3 and omega-6 based products (in particular microbial oils) to be used by food and infant formula customers. Between 2007 and 2010, Mr Dueppen worked at Microbia Inc as Director of Process Development, leading a team of engineers and chemists to develop solvent extraction, separation, purification, crystallization, and recovery processes of carotenoids.
12. Second, Dr James Wynn who gave evidence from the perspective of the Skilled Microbiologist, a person skilled in upstream processing. Dr Wynn has over 25 years of experience in industrial biotechnology and studying the production of microbial oils. In his current role as Head of Global Research & Development at AB Mauri, Dr Wynn leads a global team of specialist centres that focus on microorganism strain selection and development to provide novel and improved products that are used in the Food and Beverage Industry. He is also a contributing author to the textbook “Single Cell Oils” (including the 2005 and 2010 editions).
13. Dr Wynn’s first degree was in Applied Biology at the University of Cardiff. He completed a PhD in Applied Biology at the University of Hull in 1993, before starting post-doctoral positions at the Universities of Nottingham and Hull. He remained in the latter position until 2001, studying the biosynthesis of PUFAs in oleaginous microorganisms, including fungi and microalgae, alongside Professor Colin Ratledge (a prominent figure in the field of single cell oils).
14. Between September 2001 and September 2005, Dr Wynn worked as a Senior Scientist at Martek, leading the molecular biology team which was researching the biochemistry of key DHA producing microorganisms. That included work on Martek’s original DHA biotechnology, DHASCO oil. Later, in 2005, Dr Wynn was promoted to Principal Scientist and led a research collaboration with DSM for the development of the fungal fermentation process for the production of ARASCO. Dr Wynn left Martek in May 2009 to join the Michigan Biotechnology Institute and in 2016 joined AB Mauri in his current role.
15. Mara called a single expert witness, Dr David Kyle. Dr Kyle completed a degree in marine biology in 1974 at the University of Victoria in Canada. He obtained his PhD in 1980 from the University of Alberta on the biochemistry of plant lipids. Until 1984 he conducted post-doctoral studies at Michigan State University in plant molecular biology and biochemistry using single cell algae as model systems, which included work in institutes in Paris, Tokyo and Colchester. In 1984, Dr Kyle spent a year as a Research Scientist with Lockheed Martin working with NASA on designs for self-contained life support systems for orbiting space labs and lunar colonies. In 1985, he and the rest of the biotech team founded Martek, with the purpose of studying metabolites from algae and their potential use as foods, pharmaceuticals and diagnostics. At Martek he was initially Director of Business Development and Research Manager, and then Senior Vice President of Research and Development, responsible for directing research concerning microbial oil production and the utilisation of omega-3 and omega-6 PUFAs. He was heavily involved in the development of Martek’s Designer Single Cell Oils program, which produced oils from the microorganisms Crypthecodinium and Mortierella marketed under the names DHASCO and ARASCO. He had primary responsibility for interacting with the external commercial partners, researchers and clinicians who wanted to know more about, or obtain samples of, the oils. Professor Ratledge has credited Dr Kyle with the “major breakthrough” of recognising that microorganisms could be the key to providing a supply of DHA, and of identifying a potential microbial source at Martek.
16. In 2001, Dr Kyle left Martek and founded and served as President and CEO of Advanced BioNutrition Corporation, where the focus was on the use of extracted bio-meal as animal feed. Like Dr Wynn, Dr Kyle is a contributing author to the 2005 and 2010 editions of the textbook “Single Cell Oils”.
17. In 2008 he retired from full time work in the microbial oils field. In 2009, he was inducted into the US Space Technology Hall of Fame for his contributions to science and industry, especially for the development and commercialisation of DHASCO and ARASCO and their incorporation into infant formulas. Dr Kyle then formed his own consultancy company, and in 2013 joined the academic founders of Evolve Biosystems in Davis, California and served as Managing Director, then CEO and subsequently Executive Chairman, until the company was reorganised and renamed Infinant Health early in 2022.
My assessment of the Experts
Mr Dueppen
18. DSM submitted that Mr Dueppen was a patently honest witness who gave fair and measured answers to all the questions he was asked. For their part, Mara had no criticism of him whatsoever, agreeing that he was fair and balanced in his approach. Subject to the points which I address below (at [43]-[45]) and which concern or stem from the approach which DSM’s experts were invited to take (therefore not really their fault), I largely agree.
Dr Wynn
19. DSM submitted that Dr Wynn was a model expert witness. Mara was critical only of some isolated parts of his evidence, including in particular his evidence relating to a literature search. Mara submitted that, subject to what they characterised as ‘that significant lapse’ and a couple of other minor instances, he was trying his best to assist the court fairly on the matters which fell on his side of the split between himself and Mr Dueppen. I consider Mara’s points of criticism below, but in general, but subject to the same qualification I mentioned for Mr Dueppen, I agree that Dr Wynn was a good witness.
20. It was not surprising that Mara made only very limited criticisms of Dr Wynn and none of Mr Dueppen because Mara’s case in closing inevitably relied very heavily on their evidence - inevitably because of what became apparent in the cross-examination of Dr Kyle.
Dr Kyle
21. Despite Dr Kyle’s very considerable experience in this field, I regret to say that his evidence proved unsatisfactory in a number of respects. By way of example, I gained the impression that Dr Kyle had not adequately grasped the need to eliminate hindsight when considering the important issue of obviousness, despite setting out the Pozzoli approach in his evidence. Equally, he did not appear to have properly understood the concept of common general knowledge or the need to avoid speculation. However, it does not appear to be necessary to set out all the respects in which DSM were critical of his evidence because Mara accepted that overall his evidence and approach was shown to be unsatisfactory in several respects. Accordingly, Mara did not seek to persuade the Court to prefer Dr Kyle’s evidence over that of the DSM expert witnesses.
22. In some cases, such a concession would be fatal. Mara faced up to the problem caused by Dr Kyle’s evidence, contending that they could establish their case on each patent relying on the evidence of Mr Dueppen and Dr Wynn - this was particularly the case regarding EP155.
23. Having said all that, there remain parts of Dr Kyle’s evidence which were not challenged, both in his written evidence and on points elicited by DSM in their cross-examination of him. However, where his evidence was disputed, I will not rely on it unless either Mr Dueppen or Dr Wynn effectively agreed with the point in the course of cross-examination.
24. Before leaving the topic of the expert evidence, I should briefly outline how the expert evidence developed, particularly during the trial.
25. The initial exchange of expert evidence took place on 3 July 2024, with reply reports on 21 August. On 10 September 2024, Wynn 3 was served, responding to certain points in Kyle 2. Dr Kyle then responded to Wynn 3 in Kyle 3 dated 19 September 2024.
26. The cross-examination of Mr Dueppen started on Day 2 - Wednesday 2 October and on that day he was cross-examined as to how the Skilled Team could tell the difference between a crude oil and a refined oil (see further below). Evidently in response to that, on 3 October, Powell Gilbert posed a question to Dr Wynn which he addressed in Wynn 4, dated 4 October 2024.
27. Mr Dueppen’s cross-examination concluded during the morning of day three (Thursday 3rd October), whereupon we had to break until Monday 7 October. That day started with DSM’s application to rely on a new expert report from Dr Wynn (Wynn 4) and I heard lively argument on that issue. The arguments were very evenly balanced, so I reserved my ruling. Pending that Dr Wynn was cross-examined on Wynn 1-3. My decision to admit Wynn 4 was communicated to the parties early on Tuesday 8th. Mara had shown Wynn 4 to Dr Kyle and he had managed to draft a short report from Dr Kyle in response - Kyle 4. In order to make the best use of time, and as agreed, the cross-examination of Dr Wynn concluded on Wynn 1-3, whereupon I gave my reasons for admitting Wynn 4 whilst Dr Wynn was given the opportunity to consider Kyle 4. Dr Wynn then went back into the witness box and was cross-examined on Wynn 4.
28. There are two issues to resolve regarding the Skilled Person/Team. The first was a curious dispute debated between the parties over the Skilled Team but one which has ramifications as to the approach to prior art. The second issue was not identified or tackled directly, but it emerged from the differing approaches taken to certain of the prior art. It concerns whether the Skilled Team only operated and were interested in commercial (i.e. industrial) scale processes. I address these issues in turn.
29. The relevant law is settled: although the skilled person/team is a hypothetical construct, its composition and mindset is founded in reality. As Henry Carr J. said in Garmin v Philips [2019] EWHC 107 (Ch) at [85](v), the combined skills (and mindsets) of real teams are what matters.
30. It was common ground that the skilled person / team involved in the production of microbial oils would have skills relating to:
i) upstream processing, which covers strain selection, media development and process design to enable the production of a lipid rich biomass at a commercial scale; and
ii) downstream processing, which covers cell inactivation onwards (i.e. lysis, extraction of the oil from the cells, recovery of the crude oil, and refining the oil).
31. Although the first dispute was framed as whether a single skilled person would cover both areas, in fact the dispute between the parties appeared to be over the degree of communication and interaction between the upstream and downstream sides.
32. Dr Wynn and Mr Dueppen explained that upstream and downstream processing are distinct areas - in practice they may even be in physically distinct locations. Although the separate teams may work closely together, in their view both areas would not fall within the expertise or interest of a single skilled person. In their view, upstream processing is the province of a person with expertise and relevant experience in the field of microbiology (a “Skilled Microbiologist”), while downstream processing is the province of person with expertise and relevant experience in bioprocess engineering (a “Skilled Bioprocessing Engineer” or “SBE”).
33. In contrast, Dr Kyle considered that a single skilled person would have expertise in both upstream and downstream processing. He explained that such a person would have several years’ industry experience generally in the field of microbial oils. Dr Wynn and Mr Dueppen disagreed that such persons exist.
34. DSM acknowledged first, that many of the core CGK concepts were similar regardless of the makeup of the skilled team / person, in particular those relating to the fundamental principles of chemistry and biology and second, that in many instances it was not material if an issue is considered from the perspective of one of the members of the skilled team, as described by Dr Wynn and Mr Dueppen, or a single person, as described by Dr Kyle. In those contexts, it appears DSM were content to refer simply to the Skilled Team.
35. However, DSM made their position clear that the disagreement on this issue could affect the correct approach to obviousness and the prior art. Their point was that it is wrong in law and unfair to a patentee to consider issues such as inventive step from the perspective of a “super-skilled individual” as posited by Mara, rather than a notional team that reflects the make-up of real teams as described by Dr Wynn and Mr Dueppen. In such contexts, DSM indicated they would use the experts’ nomenclature of Skilled Microbiologist and Skilled Bioprocessing Engineer to describe the respective upstream and downstream members of the team.
36. I will keep all these points in mind, particularly as to DSM’s warning that Mara’s case required a ‘super-skilled individual’.
37. That leads me to the second issue. There is one respect in which I considered DSM’s characterisation of the Skilled Team to be too limited. DSM appeared to me to be focussed almost exclusively on processing at commercial scale, with the members of the team apparently only being interested in and only having experience in processing at commercial scale. This seemed to me to be unrealistic, not least because any commercial processing would have to have been scaled up from much smaller and experimental processes, at least some of which would have to take place at very small scale in a laboratory i.e. experiments at beaker scale.
38. I do not believe that the Skilled Team would only have experience of processing at commercial scale, or be limited, in effect, to technicians whose job is to keep the commercial processes running. In my view, they would also have experience of experimenting in the lab and then scaling up. They would have the (non-inventive) ability to develop processes, not just run them.
39. This is confirmed by consideration of the Patents and many of the Examples in them which, in the main, reflect small-scale laboratory experiments. Implementing any of the Patents would require trials in the lab for proof of concept followed by scale-up experiments as necessary.
40. This point is also confirmed by the extracts from “Single Cell Oils; Microbial and Algal Oils”, edited by Zvi Cohen and Colin Ratledge (‘the SCO Book’) which were put to Mr Dueppen in cross-examination, and by the identity and background of the authors of chapters in that book. I should explain the SCO Book was published in 2005 but arose from a symposium in May 2003 organised by Dr Kyle ‘that covered many of the on-going projects in this area’. Both sides agreed the SCO Book reflected the CGK at May 2002.
41. The SCO Book shows that there was an active (if relatively small) community of people working around the world both in commercial companies and in academia, researching microbial oils and processes to obtain them. Perhaps the best example from the academic side is Dr Barclay, but I should also mention Professor Colin Ratledge and Zvi Cohen, the editors and co-authors of the SCO Book.
42. In this regard, I refer to Chapter 13, entitled ‘Down-Stream Processing, Extraction and Purification of Single Cell Oils’ by Colin Ratledge (University of Hull), Hugo Streekstra (DSM), Zvi Cohen (Ben Gurion University) and Jaouad Fichtali (Martek). Whilst in large part this Chapter considers downstream processing at commercial scale, on p240 there is specific mention of extracting the oil from small samples of the biomass taken from laboratory fermenters. The combination of the authors of this (and other Chapters in the SCO Book) also confirms the sort of links between academia and industry that one would expect to exist.
43. This brings me to the qualification I have about the evidence of Mr Dueppen and Dr Wynn. I gained the impression that their focus was very much on the commercial scale production processes with little or no attention being paid to anything outside that. If there is any fault involved, it lies not with the experts but with the topics they were invited to cover i.e. those instructing them. It is evident that it suited DSM’s case to focus on large scale commercial processing.
44. This focus was also promoted by DSM’s adoption of the division between upstream and downstream processing. Whilst the adoption of these terms was legitimate, it needs to be kept in mind that reference to them tends to put one in mind of commercial processing. The use of these terms suited DSM’s focus.
45. However, as I discuss below, answers given by both Mr Dueppen and Dr Wynn in cross-examination served to prove that enzymatic lysis and other non-mechanical lysis methods were CGK because they would be used and explored at small scale in the laboratory, either in industry or in academia. I also gained the impression that both Mr Dueppen and Dr Wynn had a tendency to stick to the party line, as expressed in their written evidence. However, for the most part, I can rely on what Mr Dueppen and Dr Wynn said in their live evidence.
46. Finally, having said all that, the focus on commercial processing appeared to be consistent with the evidence as to the commercial barriers facing any new entrant (see [174] below).
THE COMMON GENERAL KNOWLEDGE
47. Before moving to what was agreed CGK (and the areas of dispute), I consider it is necessary to say something about the sources of CGK in this field, on which there was broad agreement between the experts.
48. As Dr Kyle observed, there was no standard textbook in the microbial oils field. Such reference books as existed arose from various symposia, typically organised by the AOCS. Dr Kyle referenced two books, with which the Skilled Team would be familiar: first, “Industrial Applications of Single Cell Oils” (1992) edited by himself and Colin Ratledge, and second, the SCO Book. Although this was published in January 2005, it arose from a symposium which Dr Kyle organised for the AOCS in May 2003 and, as noted in the preface, also echoes two earlier AOCS conferences, the first being a 1982 conference held in Toronto and the second being the AOCS conference from May 1992 in Chicago. Between them, the experts exhibited 7 chapters from the SCO Book and I was supplied with a hard copy of the book itself, a relatively slim volume of some 250 pages. The experts also referenced certain papers.
49. Thus, it appears that the primary source of CGK were symposia and conferences, with certain of the topics discussed being then recorded in the books Dr Kyle referred to. Experts on both sides also referenced certain journals. Having referenced the SCO Book, Dr Wynn said:
‘I also believe sources of CGK for the Skilled Microbiologist would have included key lipid-related developments published in the following journals: Lipids, Applied Microbiology, World Journal of Microbiology and Biotechnology, Advances in Applied Microbiology, Applied and Environmental Microbiology, Nature Microbiology, and Nature Biotechnology. The Skilled Microbiologist may have attended conferences such as the AOCS Annual Meeting, International Conference on Algal Biomass, Biofuels and Bioproducts, Algae Biomass Summit and the Annual Meeting of the Society for Industrial Microbiology and Biotechnology.’
50. Mr Dueppen also made an important point about the effect of commercial companies keeping specific details of their processes confidential, so that reference was made to regulatory submissions and published patent filings:
‘The basic scientific principles underpinning the work of the Skilled Bioprocessing Engineer would be found in standard university chemistry, biochemistry and bioprocessing textbooks. More specific, industrially applicable techniques would also be taught at various continuing education courses such as those by the American Institute of Chemical Engineers (AIChE) and the American Chemical Society (ACS). These organizations host conferences and events at which industry-specific knowledge relevant to the field of extraction of oil from
microbes and plants was discussed, although typically at a high level as many companies kept the more specific details of their processes confidential as trade secrets or until they were patented. For the same reason there were relatively few pieces of academic literature addressing more industry-specific issues facing the Skilled Bioprocessing Engineer. In addition the key companies in the field kept informed of others’ activities, including via their regulatory submissions and patent filings.’
51. Mr Dueppen also referred to the SCO Book as the most comprehensive summary of the academic and non-confidential industrial work in the field and as a good source of CGK. So there was clear agreement that the SCO Book is representative of the CGK at the EP 155 Priority Date. However it remains the case that, in this field, a lot of the CGK was not recorded in any textbook.
52. The remainder of this section is based on the Statement of Agreed CGK provided by the parties. I was told that the agreed points are not affected by the parties’ different positions on the Skilled Team. The Statement of Agreed CGK started with a section containing a series of definitions of some key terms, and then proceeded with three further sections, each describing the agreed CGK at each of the three priority dates. The parties identified a number of disputes. It is convenient to decide the minor ones as I proceed through each section.
53. Although the CGK does not change a great deal between the various priority dates, I prefer to set out the CGK (and decide the disputes) at each priority date in the context of each patent.
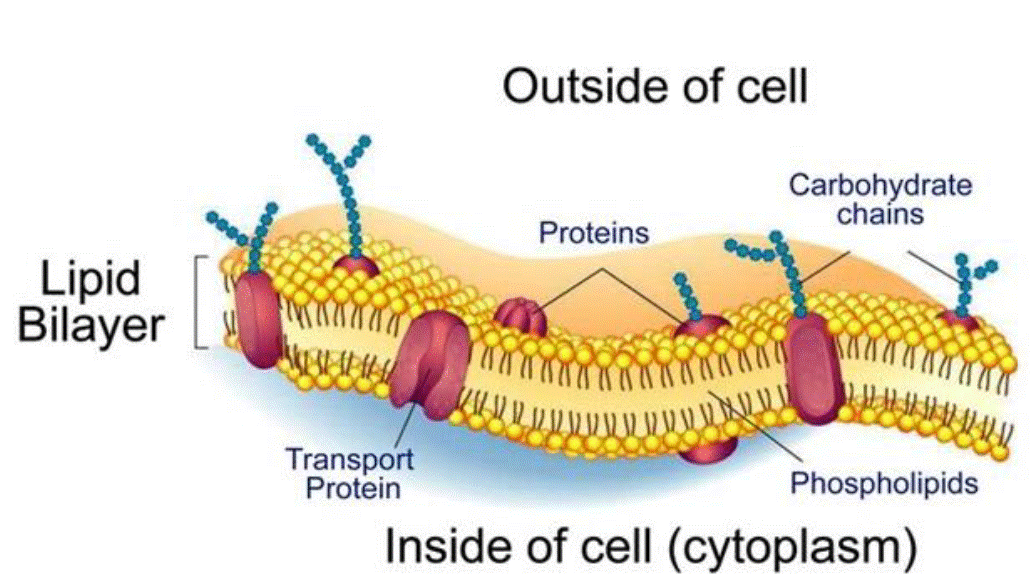
54. AOCS (the American Oil Chemists’ Society) - an international professional organisation founded in 1909 and based in Urbana, Illinois for those involved with the science and technology related to fats, oils, surfactants, and other related materials;
55. Arachidonic acid - often abbreviated to ARA, is a polyunsaturated omega-6 fatty acid, often also referred to as 20:4, 20:4 n-6, or 20:4 ω-6;
56. ARASCO® or Arachidonic Acid Single Cell Oil - a single cell oil developed by Martek from the fungi Mortierella alpina which contains primarily only one PUFA, arachidonic acid, in its fatty acid profile;
57. DHASCO® or Docosahexaenoic Acid Single Cell Oil - another single cell oil developed by Martek from the algal species Crypthecodinium cohnii which contains primarily only one PUFA, docosahexaenoic acid, in its fatty acid profile;
58. Docosahexaenoic acid - often abbreviated to DHA, is a polyunsaturated omega-3 fatty acid, often also referred to as 22:6, 22:6 n-3 or 22:6 ω-3;
59. Docosapentaenoic Acid - often abbreviated to DPA, is a polyunsaturated fatty acid, often referred to as 22:5. There are both omega-3 and omega-6 forms of DPA referred to as 22:5 n-3; 22:5 ω-3 and 22:5 n-6; 22:5 ω-6 respectively;
60. Eicosapentaenoic acid - often abbreviated to EPA, is a polyunsaturated omega-3 fatty acid, often also referred to as 20:5, 20:5 n-3 or 20:5 ω-3;
61. GRAS or Generally Recognized As Safe – the status given by the United States Food and Drug Administration to a chemical or substance added to food if it is considered safe by experts under the conditions of its intended use;
62. Phospholipids - a class of lipids which have a molecular structure comprising a hydrophilic "head" containing a phosphate group and two hydrophobic "tails" derived from fatty acids;
63. Polyunsaturated Fatty Acids – often abbreviated as “PUFAs” - are a type of fatty acid characterised by a backbone having two or more carbon–carbon double bonds. They can be characterised as omega-3 PUFAs or omega-6 PUFAs depending on the position of the double bonds;
64. Structural Lipids - those lipids that comprise the lipid bilayers in the cell membrane and cellular organelles. Structural lipids are primarily phospholipids.
65. Storage Lipids – those lipids which provide energy to a cell. Storage lipids are primarily triglycerides;
66. Triglycerides (more correctly referred to as triacylglycerides but often also abbreviated to “TAG” or “TG”) - a class of lipids consisting of three fatty acid chains linked by a glycerol backbone.
67. The following matters were agreed CGK at the EP155 Priority Date namely 3 May 2002.
68. Lipids are an essential part of the human diet. In chemical terms, lipids are defined very broadly, according to their chemical properties rather than their structure. The definition of a lipid encompasses a huge variety of chemicals including sterols, carotenoids, polyhydroxylalkanoates, triacylglycerols (TAGs, also referred to as triglycerides, TGs or neutral lipids), and phospholipids (also referred to as polar lipids). Fatty acids can be considered the structural units of fats or lipids, in much the same way as amino acids are the structural units of proteins. In some lipids (for example, TAGs and phospholipids), the fatty acids are covalently bonded to a glycerol backbone via an ester linkage that is shown in the orange box in Figure 1 below.
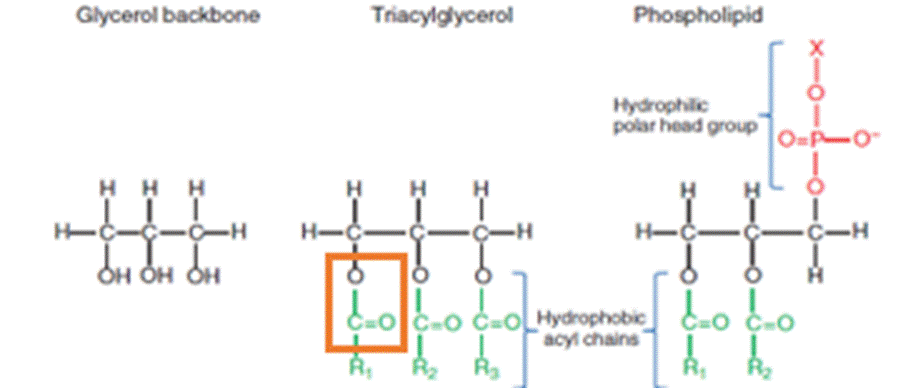
Figure 1: The major forms of fatty acids in cells
69. There are a large variety of fatty acids that differ in their chemical structure and, therefore, in their chemical characteristics. The fatty acid composition, often referred to as the fatty acid profile, of a lipid has a large impact on its physicochemical properties. Fatty acids play key roles in the function of lipids in biological cells and certain fatty acids play vital roles in human and animal development and health. Fatty acids, and the lipids they form, also have commercially significant applications in the food industry (cooking oils/fats, cocoa butter, etc.) and chemical industry (detergents, surfactants and lubricants).
70. Fatty acids are simple lipids, and consist of a nonpolar (‘fatty’) hydrocarbon chain terminating in a polar carboxylic acid (carboxyl) group). Generally speaking, fatty acids having fewer than 6 carbon atoms in the hydrocarbon chain are referred to as short chain fatty acids. Medium chain fatty acids are those with hydrocarbon chains of from 6 to 10 carbons, long chain fatty acids are those having 12 to 18 carbons in the hydrocarbon chain and very long chain fatty acids have hydrocarbon chains of 20 or more carbon atoms. Once the carbon chain length exceeds approximately six carbons, then the hydrophobic nature of the hydrocarbon chain dominates the hydrophilic properties of the polar carboxylic acid group, and the molecule overall takes on the hydrophobic characteristic of lipids (i.e. being insoluble in water).
71. With minor exceptions, the fatty acid residues in lipids are straight acyl chains with a carboxylic acid group at one end and a methyl group at the other end. The structure of fatty acids can vary in any one (or more) of three ways:
i) the number of carbon atoms in the carbon chain;
ii) the number of carbon–carbon double bonds (C=C) they contain; and/or
iii) the position of these double bonds.
72. Some example fatty acids to illustrate this variation are shown below:
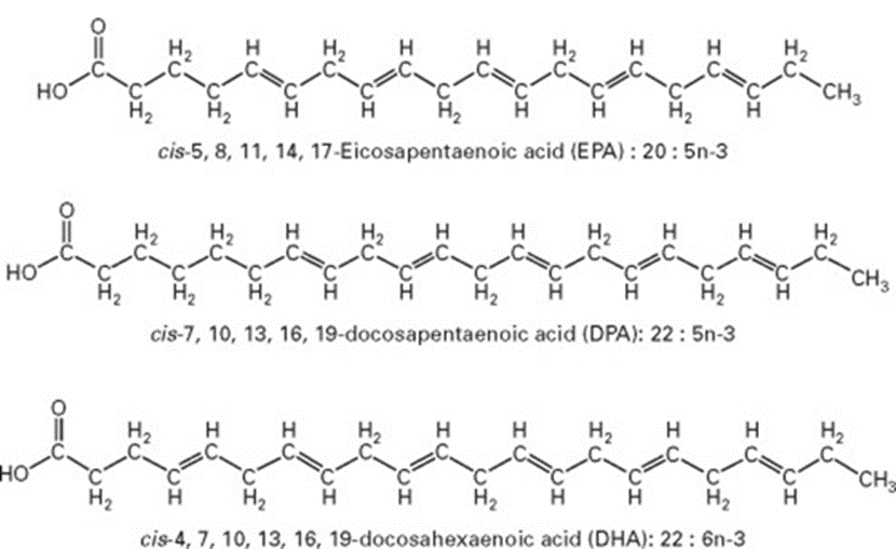
Figure 2: The chemical structure of certain fatty acids
73. As the length of the fatty acid increases, the melting point of the fatty acid tends to increase. Conversely, the addition of double bonds into the fatty acyl chain tends to cause the melting point of the fatty acid to decrease. If the fatty acids contain no carbon-carbon double bonds (C=C) they are known as ‘fully hydrogenated’ or ‘saturated’ fatty acids, in contrast, ‘unsaturated’ fatty acids have one or more double bonds in the hydrocarbon chain. Fatty acids with hydrocarbon chains containing two or more double bonds are also referred to as polyunsaturated fatty acids (PUFAs).
74. PUFAs can exist in a number of molecular forms, including: (i) as a free fatty acid; (ii) as an ester; (iii) as a phospholipid and (iv) as a triglyceride (a.k.a. triacylglyceride, triacylglycerol, TG or TAG).
75. Phospholipids are comprised of a glycerol molecule wherein one of the hydroxyl groups is covalently attached to a polar phosphate group and the remaining two hydroxyls are esterified to fatty acids. Because of the presence of the polar phosphate-containing head group, phospholipids are not entirely hydrophobic but display amphiphilic properties. Amphiphilic refers to the fact that phospholipids possess a polar region that is hydrophilic (attracted to water) and a region that is nonpolar and hydrophobic (repelled by water).
76. The amphiphilic nature of phospholipids allows them to perform a vital role in all cells, as the membrane which separates the cell’s intracellular components (DNA, RNA, proteins etc.) from the outside aqueous environment is comprised primarily of a phospholipid bilayer. The phospholipids self-align into a bilayer with the hydrophobic parts in close contact forming the hydrophobic inner core of the membrane and the hydrophilic ends (phosphate groups) being attracted to the aqueous environments both outside and inside the cells. Figure 3 illustrates the lipid bilayer cell membrane.
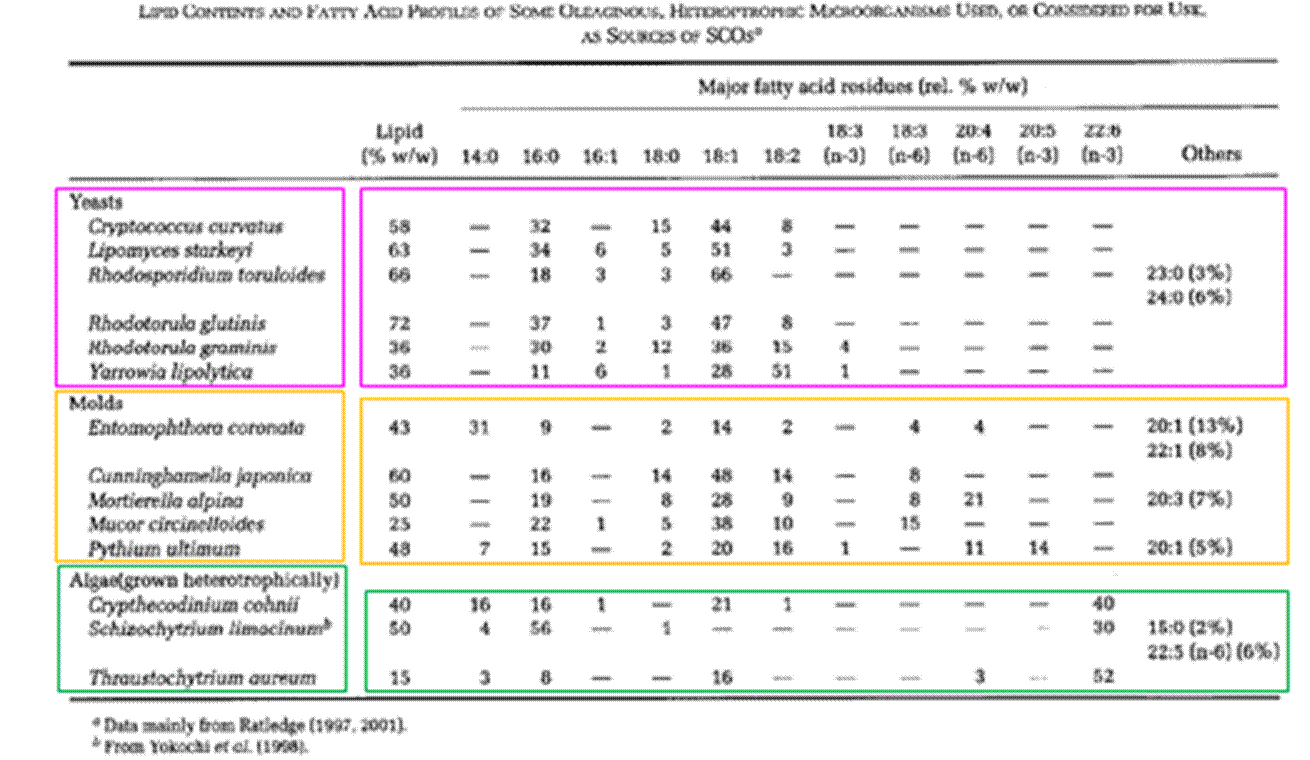
Figure 3: Diagram of a cell membrane comprising a phospholipid bilayer
77. The other dominant form of fatty acyl lipids in cells are TAGs, having three fatty acid residues esterified to the glycerol backbone (see diagram in Figure 4 below, where each position on the glycerol backbone is typically labelled sn-1, -2 and -3). TAGs are hydrophobic molecules which will coalesce with other TAG molecules to form an oil droplet (oil body) within the cell. Glycerols having only one or two fatty acid ester substituents are similarly known as mono- and di-glycerides respectively.
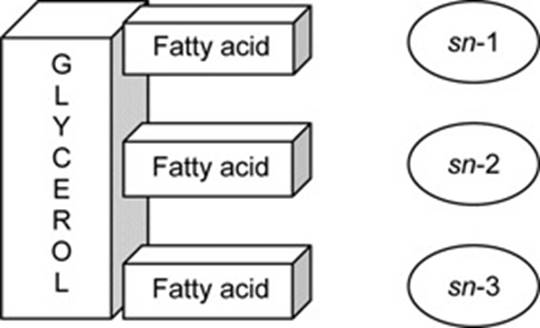
Figure 4: Block diagram of a TAG
78. In contrast to phospholipids, in most cells, TAGs serve no specific physiological function (e.g. they do not form cell membranes), but they do act as storage molecules. TAG is particularly suited to the role of an energy and carbon store as it is a very concentrated form of energy; fat stores approximately twice the energy per unit mass as protein or starch.
79. Triglycerides (along with very small quantities of diglycerides and monoglycerides) are the predominant constituents of oils and fats of commercial importance. Indeed, most of the lipids we consume daily as part of our diets are in triglyceride form, either as a liquid oil (e.g. vegetable oil) or as a solid fat. Commercial oils are generally devoid of phospholipids as they are removed during refining (as discussed below).
80. The naming of fatty acids can be a somewhat confusing topic, as a number of names derived in different ways can be (and routinely are) given to any given fatty acid. In many cases, these names are used interchangeably, even within the same document. The table below includes a summary of the systematic, common and numeric names of some of the fatty acids relevant to these proceedings:
|
Systematic name |
Common name |
Abbreviation |
Numerical designation |
|
(all-cis)-5,8,11,14-eicosatetraenoic acid |
Arachidonic Acid |
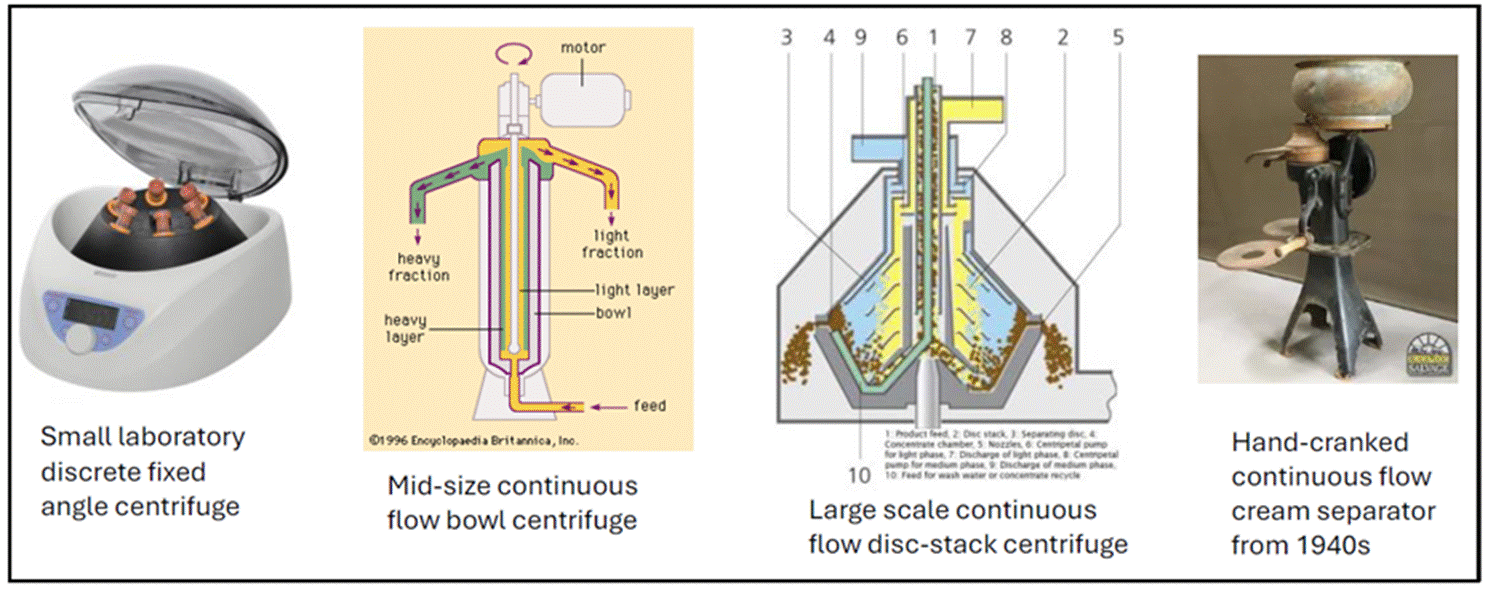
ARA |
20:4 n-6 |
|
Eicosapentaenoic acid |
Timnodonic acid |
EPA |
20:5 n-3 |
|
Docosapentaenoic acid |
Clupanodonic acid |
DPA |
22:5 n-3 and
22:5 n-6 |
|
Docosahexaenoic acid |
Cervonic acid |
DHA |
22:6 n-3 |
|
Heptadecanoic acid |
Margaric Acid |
HDA |
C:17 |
81. The numerical designation takes the form X:Y n-Z, where X is the number of carbons in the acyl chain, Y is the number of double bonds contained in the acyl chain, and Z is the number of carbons from the last double bond to the terminal methyl group (inclusive) and therefore informs the reader which series (n-3, n-6 or n-9) the fatty acid belongs to.
82. Fatty acid methyl ester analysis, also known as FAME lipid analysis, is a method used to identify and quantify the fatty acid composition of lipids in biological samples. The process involves extracting lipids from a sample, converting them from more complex phospholipids, mono-, di- or triglycerides into simple methyl esters of the individual fatty acids through a chemical hydrolysis reaction, and then analysing the resulting methyl esters using techniques such as gas chromatography.
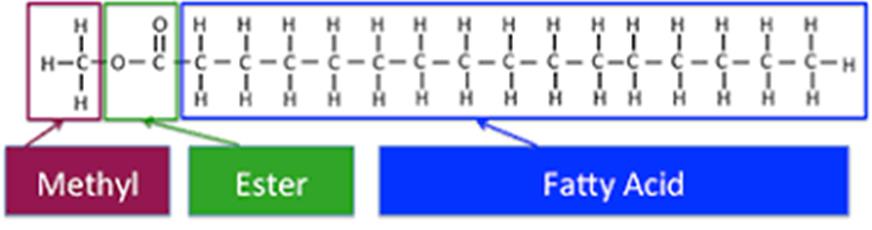
Figure 5: Molecular structure of a FAME
83. In most cells (animal, plant, and microbial), fatty acids are synthesised through the action of an enzymatic complex called fatty acid synthase. The building blocks of fatty acids are molecules containing 2 carbons (acetyl-CoA) and, as such, the majority of fatty acids found in nature possess even numbers of carbon atoms in their backbone, as the fatty acid synthase enzyme acts by repeatedly joining these two carbon blocks together. There are some microorganisms that utilise a 3-carbon molecule (propionyl-CoA) as a building block, which means the resulting chain can have an odd number of carbon atoms, although such microorganisms are rare.
84. Eukaryotes (i.e. organisms having cells containing a nucleus, which include animals, plants, fungi and many unicellular organisms) primarily synthesise PUFAs by an oxygen dependent reaction that involves the elongation and desaturation of the shorter fatty acids. Humans and animals are capable of synthesising certain PUFAs with chain lengths from 18 to 22 carbons, however they generally do not possess several key enzymes to allow the production of certain other key long chain PUFAs described as essential fatty acids. In particular, the longest and most unsaturated fatty acids commonly found in nature, eicosapentaenoic acid (EPA), 20:5 and docosahexaenoic acid (DHA), 22:6 are only produced in small amounts by animals and humans. As a result, preformed EPA and DHA are important nutrients for most animal species and humans. DHA and EPA are omega-3 fatty acids (n-3/ω-3), and the molecular structures are shown in Figure 2 above.
85. The production and use of olive oil as a part of the human diet dates back more than 3 millennia. It has also been apparent throughout history that oils and fats are useful sources of metabolic energy. By the 1950s, research into fatty acids as a source of nutrition had become more focused on the importance of the degree of unsaturation, and since the 1960s, dietary recommendations have consistently advised reducing total dietary fat, and replacing saturated fats with unsaturated fats (i.e., vegetable oils). By the mid-1980s the growing recognition of the cardiovascular health benefits associated with PUFAs (especially EPA and DHA) found in fish oil, led to a major expansion of fish oil supplement sales in health food stores and pharmacies around the world, as discussed below.
86. Another important essential fatty acid is arachidonic acid (ARA). ARA is an omega-6 fatty acid (n-6/ω-6) PUFA with 20 carbons but only 4 carbon-carbon double bonds, and the molecular structure of ARA is shown in Figure 6 below.
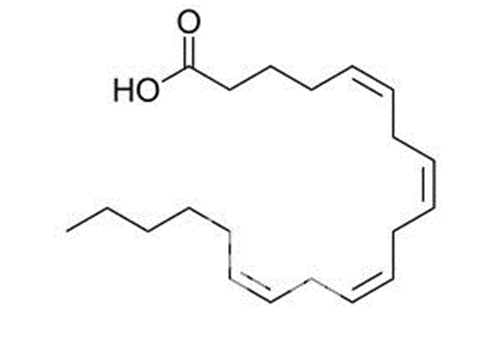
Figure 6: Molecular structure of ARA
87. EPA is a precursor to anti-inflammatory compounds (eicosanoids and prostaglandins) that support cardiovascular health. DHA is the predominant polyunsaturated fatty acid in the brain and retina and is found in high concentrations in heart tissue. DHA was known to be important for infant development, alongside ARA, and both these fatty acids had been shown to be present in breast milk. The importance of DHA and ARA resulted in the joint FAO and WHO recommendation in 1994 that the fatty acid composition of infant formulas should correspond to the proportion of these fatty acids in breast milk, and as discussed below by 2000 DHA and ARA oils derived from microorganisms were being added to infant formula.
88. As early as the 1980s and 1990s the dietary supplements market was replete with fish oil products. Fish oil is produced as a byproduct of fish meal production. Fish meal, a major component of commercial animal and aquaculture feed is made from fish carcasses and offal after the water and oil is removed, and is a valuable source of protein, essential amino acids, vitamins, and minerals that was, and still is, used all around the world. However, the industrial production of fish meal involves process steps that can result in excessive oxidation of the fish oil and inclusion of oil-soluble contaminants, and attempts to utilise fish oils as an ingredient in food products were unsuccessful due to their strong fishy taste and odours. Furthermore, even once cleaned up and purified, a typical fish oil will only contain between 5-10% DHA and 25-30% EPA, with cold-water fish, such as tuna, salmon, mackerel, herring, and sardines, containing the highest amounts of DHA and EPA. Because of the cost of contaminant clean-up, the resulting low level of DHA, and sustainability issues, researchers began looking for alternative sources of triglyceride oils which contained higher levels of DHA, or for ways of artificially elevating the DHA content of an existing triglyceride oil.
89. It was known to be desirable to have EPA and DHA concentrations at levels higher than those naturally present in the fish oils, and there were a variety of techniques which were considered and/or had been used to increase the EPA and/or DHA concentration of a fish oil.
90. Urea fractionation and molecular distillation processes were the two well-known methods to concentrate omega-3/6 fatty acids that provided different concentrations of the fatty acids than were available naturally. Urea fractionation is a technique where saturated or monounsaturated fatty acids are trapped in crystals of urea at low temperatures, leaving behind a liquid that is highly enriched in PUFAs. Molecular distillation separates fatty acids according to their boiling points, which in turn is related to their chain length and number of double bonds, which allows fractions enriched in DHA or EPA to be obtained. Both processes work by preferentially removing undesirable fatty acids to concentrate the level of the desired fatty acid as a percentage of the total fatty acids, resulting in an enriched oil.
91. Desirable nutritional supplements in the form of structured lipids were also produced from fish oils. Structured lipids are lipids that have undergone synthetic molecular modification, either chemically or enzymatically, to alter their natural biosynthetic form, by enriching a desired fatty acid. The modifications can change the nutritional value or physiochemical properties of the starting oil, for example to increase the bioavailability of the fatty acids in the oils or enhance solubility, which are potentially desirable outcomes when manufacturing food, pharmaceutical and cosmetic formulations.
92. Although the enriched oils and structured lipid products produced from fish oils were used to manufacture more desirable oils, there were significant environmental and commercial drawbacks to producing enriched oils and structured lipids (e.g. the chemicals required and/or energy demands). These drawbacks meant there was a desire to find alternative sources of essential fatty acids that were more economical and environmentally friendly.
93. As noted above, the disadvantages of using fish oil as nutritional supplements were well understood. The key to finding an alternative source of PUFAs to fish oil came with the realisation in the early 1990s that the fish themselves do not naturally produce DHA and EPA (as they lack the key enzymes required) but instead obtain it from their diet of marine microbes, including marine algae. Marine microbes represent the primary food source for all sea life, including fish who ultimately derive most of their PUFAs from marine algae. Scientists therefore started investigating these marine algae as potential producers of PUFA rich oils.
94. Microorganisms are organisms that can only be seen through a microscope. Scientists would mainly use the scientific name of microorganisms to refer to different types of organisms. The scientific names of microorganisms are Latin based names that identify the organism based on their taxonomy. They encapsulate their genus and species. Whilst all organisms have a scientific name (e.g. humans are Homo sapiens), the scientific designation is particularly useful in microbiology as there are a large number of different microorganisms and they are often described by reference to their taxonomic ranking.
95. There are 8 levels in the taxonomic hierarchy, and below is the taxonomic hierarchy of a human for illustrative purposes:
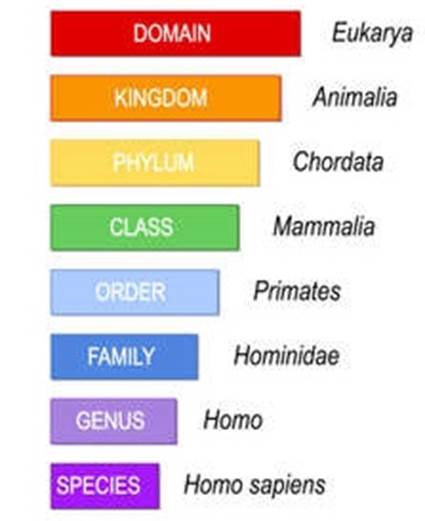
Figure 7: Taxonomic rankings
96. Taxonomy is a specialist field, but the Skilled Person/Team would understand that microorganisms are classified by taxonomists (with input from geneticists) based on morphology and genetic similarity (in combination).
97. The overwhelming majority of microorganisms have the ability to produce a range of fatty acids, predominantly as phospholipids and/or TAGs. The production of fatty acids and their incorporation into phospholipids are physiologically necessary (e.g. for cell growth) and, therefore, are part of cellular primary metabolism. In contrast, the accumulation of TAG (storage lipid) is not a physiological necessity and is not a universal feature of all living cells. Storage lipid accumulation is an example of secondary metabolism (see paragraphs 73 to 78). This means not all microorganisms accumulate significant quantities of TAG. Species which do not accumulate storage lipid are designated non-oleaginous microorganisms and contain less than 25% (dry weight) as lipid - the majority of which is phospholipid in the membranes of the cell. In contrast, oleaginous microorganisms may contain greater than 25% (dry weight) cell lipid under suitable conditions, the majority being in the form of storage TAG.
98. There had been many unsuccessful attempts to produce commercially viable microbial oils from oleaginous microorganisms dating back to the Second World War, although the possibility of microbial oils had been known since the 1890s. The key reason for the historic failure of microbial oils was the lack of large-scale fermentation technology that rendered their production too costly and difficult.
99. Heterotrophic microbial oils (i.e. those produced by fermentation, which does not require light) needed a large amount of nitrogen and carbon in order for the microorganisms to grow. For every 1000kg of oil to be produced, >10,000 litres of culture had to be grown and 5,000kg of sugar metabolised. This meant microbial oils were unable to compete with the cost and efficiency of producing oils from traditional animal and plant sources using mature methods of production and processing. Attempts to utilise autotrophic microorganisms (usually types of photosynthetic microalgae) to decrease the cost of microbial oils were actually more difficult, and have never been successfully deployed at a commercial scale. The major challenge had been that the large and open ponds necessary to expose the culture to sunlight created difficult to control environmental conditions, which can easily result in contamination. They also result in very low cell densities (<5 g/l) and high harvesting costs that make them ultimately more expensive than heterotrophic fermentation and therefore less commercially attractive.
100. The first commercially viable microbial oil was the γ-linolenic acid (18:3n-6, GLA) identified by Professor Colin Ratledge. GLA is the active ingredient in evening primrose oil, which had been historically used as a folk remedy for a range of conditions. Despite initial success when launched in 1985, the production of this microbial oil was halted after around six years when a plant alternative was identified in the form of borage oil.
101. There remained a continued interest in microbial oils, both academically and industrially. Specific microorganisms and microbial oil products are discussed below, but in summary by 2002 it was understood that:
i) The number of oleaginous microorganisms was relatively small in comparison with the total number of species.
ii) The oleaginous microorganisms were mainly species of yeast and fungi (including moulds); few bacteria produced much extractable edible oil.
iii) The oils produced by these microorganisms were similar to plant oils, mainly composed of triglycerides comprising fatty acids similar to vegetable oil, although often in different distributions relative to those found in plant oils.
iv) Some algae produced fairly high amounts of lipid that tended to be more complex in their fatty acid profile compared to those from the yeasts and fungi. However, they also contained the same fatty acids that occurred in plant oils and some PUFAs were observed to be like those found in fish oils.
v) Oil accumulation in the highest producing microorganisms could be increased by starving the cells of a supply of nitrogen (or a nutrient other than carbon). The cells generally responded to the deprivation of that key nutrient by no longer dividing and entering a lipid storage phase in which the excess carbon, still present in the growth medium, was converted into storage lipid in the form of triglyceride. If the cells were subsequently returned to a situation where nitrogen was available, the oil reserves would be quickly mobilised and rechannelled, and the cells would start growing and dividing again. Lipid accumulation was discovered to be a stress-induced response with the triglyceride oil being an intracellular energy storage material.
102. A typical profile for the accumulation of lipid in an oil-producing microorganism is shown below in Figure 8 which is taken from Chapter 1 of Single Cell Oils (first edition, 2005).
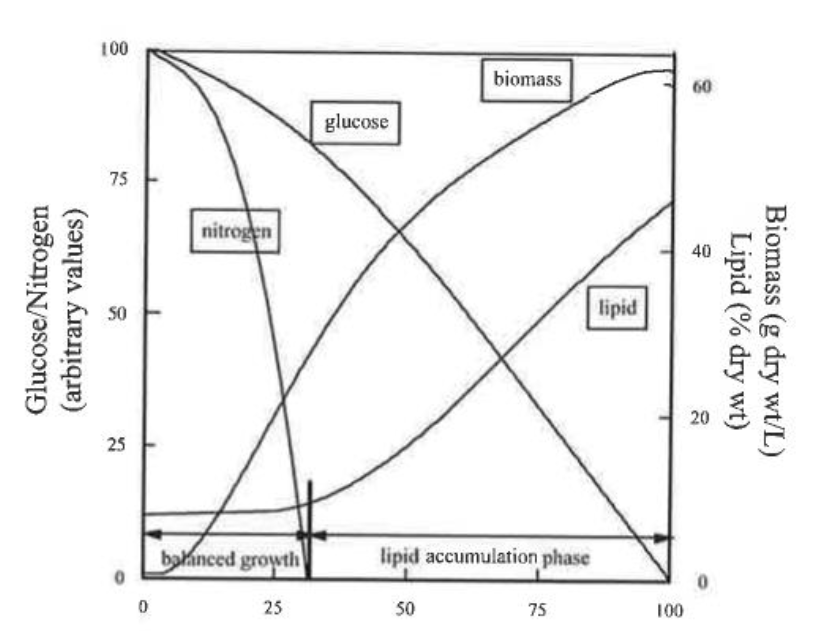
Figure 8: An idealised representation of the process of lipid accumulation in an oleaginous microorganism
103. This meant oleaginous microorganisms that produced high levels of a fatty acid of interest could be used to manufacture oils.
104. Oils produced directly from microorganisms were termed “microbial oils” or “Single Cell Oils” (“SCOs”). The annotated table from a review article dated 2002 (Ratledge and Wynn (2002) “The Biochemistry and Molecular Biology of Lipid Accumulation in Oleaginous Microorganisms” Advances in Applied Microbiology, Volume 51) shows the simple and rich fatty acid profiles of some oleaginous heterotrophic microorganisms that would have been known by that time to be in use, or considered to be used, as SCOs. The table shows the wt% of total fatty acids in the cells, alongside the wt% of individual fatty acids as a percentage of total fatty acids:
105. Yeasts are unicellular fungi and there were a number of oleaginous yeasts that were considered for use as sources of SCOs, in particular yeast oils were considered interesting as a potential source of a cocoa butter equivalent. It was considered a desirable SCO target, as it is a high value product used to make chocolate, and at the time of this product development cocoa butter prices were at a record high.
106. Moulds are fungi which grow as multicellular filaments to form a colony, and some of the most interesting moulds at the EP 155 Priority Date in May 2002 were organisms of the Mortierella genus, as species within this genus had been shown to produce substantial amounts of arachidonic acid. By 2002 an ARA oil produced from Mortierella alpina, often referred to as ARASCO, had been approved by the US regulatory body for inclusion in infant formula, together with DHA from the microorganism Crypthecodinium cohnii (discussed below). The high prices commanded in the infant formula market rendered it commercially viable to produce these essential fatty acids from a microbial source for this use.
107. There are three species of microorganisms (Crypthecodinium cohnii, Schizochytrium limacinum and Thraustochytrium aureum) identified in Table 1 at paragraph 104 above, which are grouped together as “algae”. This term was often used to describe microorganisms that were photosynthetic (or were considered to have once been photosynthetic and had lost their ability to photosynthesise at some point during their evolution). The term was used to describe oil produced from these organisms in marketing and sales literature.
108. Crypthecodinium cohnii is a marine dinoflagellate. The majority of dinoflagellates are photosynthetic. However, Crypthecodinium cohnii was identified by Martek in the late 1980s and is heterotrophic (it grows on glucose in the dark), does not contain chloroplasts, and produces no PUFA in its cell lipid in any appreciable amount other than DHA. This DHA rich oil is also accumulated primarily as triglyceride which, as noted above, is the preferred form of lipid for food use.
109. The commercialised SCO produced from C.cohnii by Martek was referred to as DHA Algal Oil or “DHASCO”. Regulatory approval for DHASCO (together with ARASCO) to be used in infant formula was granted in 2001. DHASCO was also available as an over-the-counter nutritional supplement for adults.
110. For the purposes of illustration, an electron micrograph image of Crypthecodinium cohnii cells is shown below. This shows the cells towards the end of the growth phase (when the nitrogen supply has been limited but carbon continues to be supplied). The image shows the cells packed with lipid bodies (sometimes called “fat globules” or “oil bodies”) which constitute over half the dry cell weight.
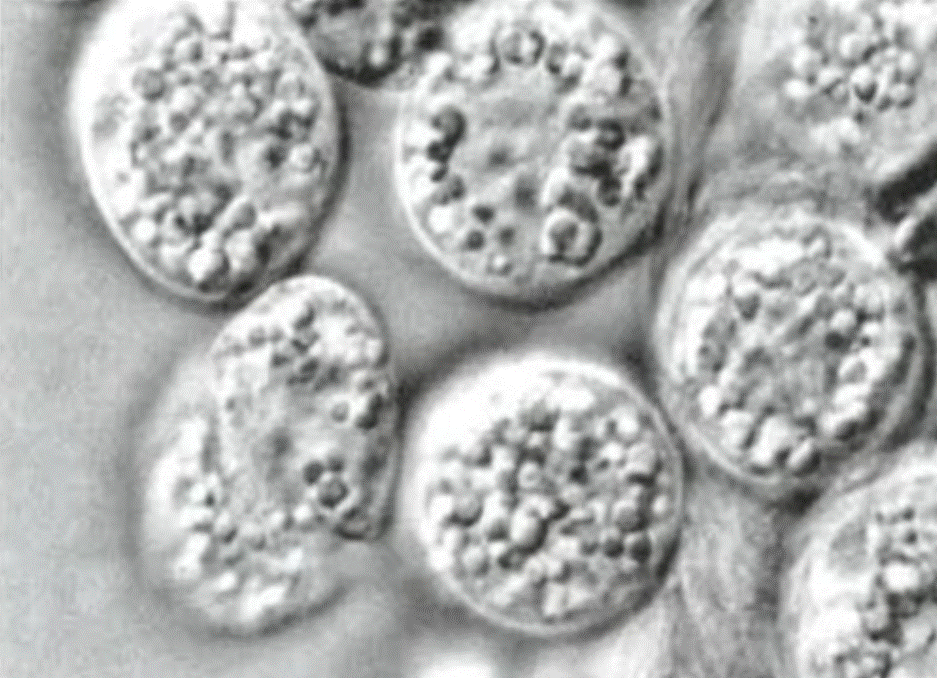
Figure 9 - C.cohnii cells with lipid bodies visible
111. Schizochytrium limacinum and Thraustochytrium aureum are closely related species of the taxonomic order Thraustochytrid. In 2002 “Thraustochytrium” and “Schizochytrium” belonged to the Thraustochytrid order.
112. Thraustochytrids were also known to produce DHA (as shown in Table 1 above), but unlike C.cohnii where the only long-chain PUFA produced was DHA, Thraustochytrids were also known to produce docosapentaenoic acid (“DPA”) (22:5 n-6). By 2002, DHA from Thraustochytrids had not been approved for use in infant formula, but the oil was sold as a nutraceutical supplement under the commercial name SeaGold (later DHAGold and subsequently S-type DHA / DHASCO-S). The biomass of the Schizochytrium produced by the company OmegaTech had been used as a supplement for aquaculture feed, poultry feed to produce eggs rich in DHA, and to produce DHA-enriched milk from dairy cows.
113. The American Type Culture Collection (referred to as “ATCC”) is a nonprofit organisation based in Virginia, USA which collects, stores, and distributes standard reference microorganisms, cell lines and other materials for research and development, including those deposited in association with filing patent applications based on those materials. The Skilled Person/Team could have obtained microbial strains which had been deposited and maintained at the ATCC (or other similar well-known culture collections). The Skilled Person/Team could also have collected and isolated their own microbial strains from nature.
114. Below is a summary of how a microbial oil was made, mainly by reference to Martek’s DHASCO oil (DHA from Crypthecodinium cohnii), using annotated figures from the Single Cell Oils book:
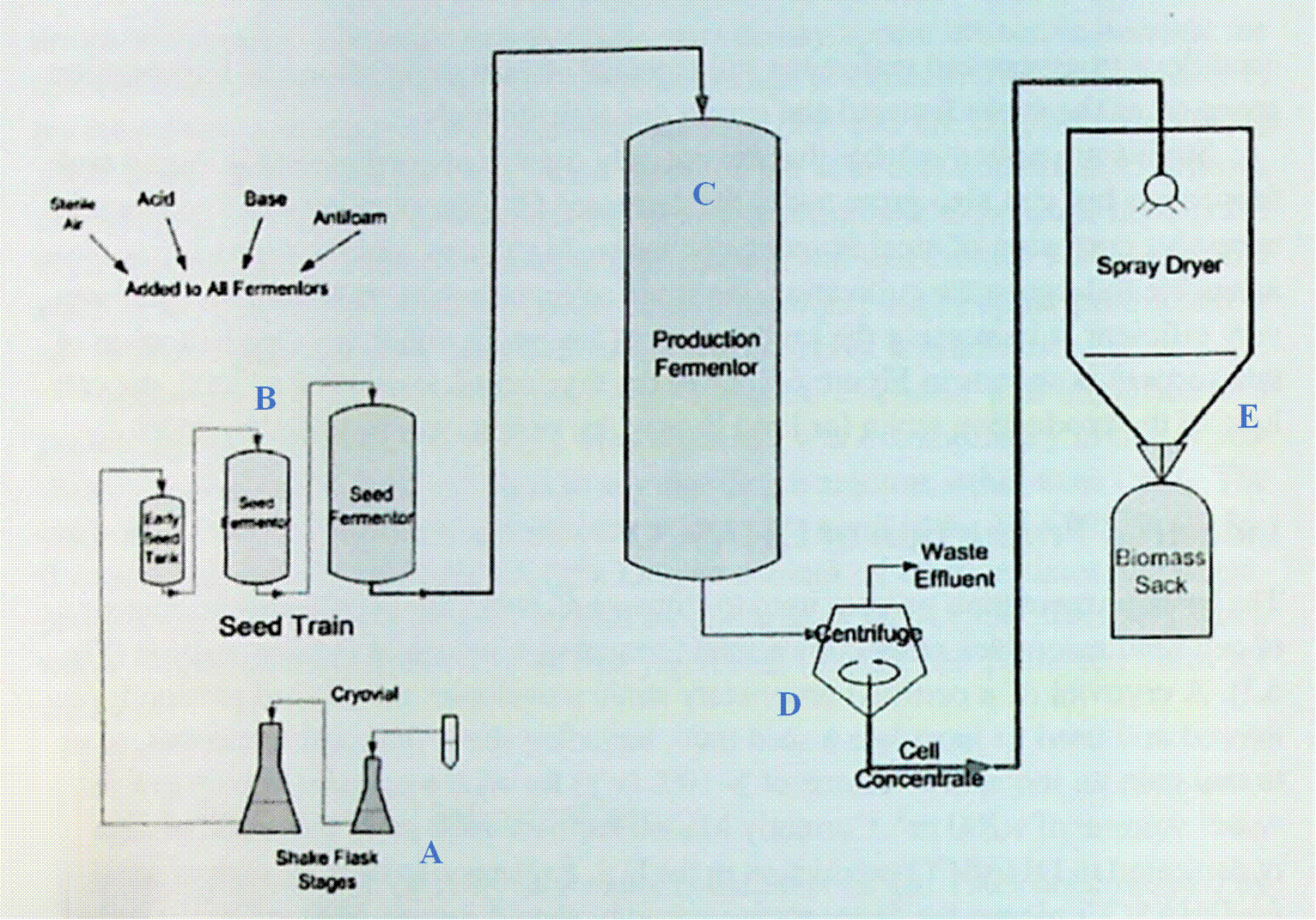
Figure 10: Overview of DHASCO microbial oil production (to drying step)
115. Steps A, B and C which have been marked on Figure 10 involve:
i) Initial inoculation - The cell line (often stored in vials at -80°C until needed) is used to inoculate small volume shake flasks containing starter media (containing nutrients for growth, and optimised in terms of salinity, pH etc.), and a series of shake flasks of increasing volume are used as the cells multiply (often referred to as the biomass increasing).
ii) Seed train - As the cells multiply, and the biomass increases further, the composition is transferred to a moderately sized seed fermenter, generally made from stainless steel, and media to encourage growth continues to be supplied. Again, usually a series of seed fermenters of increasing volume would be used. The fermentation growth media contains a low-cost carbon substrate (typically glucose) as well as a nitrogen source (typically ammonia, ammonium sulphate, yeast extract or yeast peptone) and other micronutrients and essential vitamins.
iii) Production - The growing biomass is then transferred to a larger production fermenter (e.g. 100m3, and about 10 times the size of the final seed train fermenter), again usually made from stainless steel. The fermentation tank which is used to grow the microbes is sparged with air, which provides the necessary oxygen to drive the cellular aerobic metabolism and flushes away any carbon dioxide in the exhaust. The contents of the tanks are typically mixed during fermentation to break up the sparging air bubbles, thereby improving the oxygen transfer to the cells and maximizing growth. This also helps avoid the cells aggregating (clumping together). However, these processes of sparging and mixing can in turn lead to foaming, which if left unchecked could even cause the fermenter to overflow. Consequently, an antifoaming agent is always added to the fermenter. Surfactants were (and still are) commonly used as antifoaming agents, as they decrease the surface tension thus reducing the formation of bubbles and therefore foam.
116. Under ideal growth conditions, the culture will grow exponentially and in this phase the cells are not producing significant levels of triglyceride lipids. Once the biomass has increased to target volume, the conditions are altered to encourage the accumulation of lipids (instead of the cells continuing to multiply). In particular, it was well known that limiting available nitrogen will slow down growth and, when provided at the same time with excess energy (e.g. glucose), oil producing organisms will typically switch their metabolism from growth to accumulation of oil as an energy store.
117. Next, at step D above, the biomass (microbial cells) can be heat-treated to pasteurise the cells to stabilise them, by inactivating the cell’s natural metabolic processes that may damage or consume the intracellular lipid. The biomass is then concentrated and separated from the growth media (called ‘harvesting’ the cells).
Figure 11: Examples of different types of centrifuge
118. In the production of DHASCO, this harvesting was through centrifugation. In general (i.e. not limited to the production of DHASCO), centrifugation involves spinning a solution at high speed in order to separate components of different densities through the use of centrifugal force. A simple bench-top centrifuge involves spinning test-tube like vials, leaving the lighter, less dense phase at the top of the vial and the heavier, denser phase at the bottom of the vial. On a small or laboratory scale, centrifugation was typically performed using a swinging bucket or fixed-angle centrifuge, where discrete volumes of the sample are spun simultaneously, and the supernatants and pellets are pooled after centrifugation. In large-scale operations, continuous flow centrifuges (e.g., bowl centrifuges, disc-stack centrifuges, or decanting centrifuges) were extensively used for liquid/liquid separations. In a disc-stack centrifuge, centrifugal force separates the heavier aqueous layer from the lighter oil layer in a horizontal mode, with the heavier water layer discharging farthest from the centrifuge’s central axis of rotation and the lighter lipid layer discharging closest to the central axis. Adjustable dams allow fine-tuning of the separation point between the two phases. The main advantage of continuous flow centrifugation is its efficiency; the centrifuge does not need to be shut down during harvesting. Instead of gravity, centrifugal force - ranging from 4,000 to 14,000 times gravitational force in disc-stack centrifuges - drives the separation.
119. During the centrifugation of cells carried out for the production of DHASCO most, but far from all, of the liquid media in the fermentation broth is separated off. The resulting biomass is dried as shown in step E, and spray drying was a common technique. The goal was to obtain a powder of mostly intact dried cells, as if the lipid remains within the cells it is more (although not totally) protected from the effects of oxidation. This enables the biomass to be stored for a period of time if necessary.
120. The production of sunflower oil, vegetable oil, rape seed oil, etc. was many decades old by 2002, and there was a lot of knowledge on techniques for extracting those oils, which had been proven to work on industrial scale. The process of growing microorganisms in a broth of liquid media is inevitably very different to growing plants, but the industry leveraged the very well-established oil extraction techniques from plant / seed oil production. Therefore, one aim of the drying process in step E was to obtain a solid product which could then be treated in a similar way to plant matter or seeds.
121. The first extraction techniques used for microbial oils were adapted from this decades old hexane extraction technology used in the vegetable / seed oil industries. However, hexane extraction still had certain drawbacks, as it is a hazardous substance in the factory due to its volatility and flammability. The downsides of hexane extraction were well known in 2002, but microbial oil production on a commercial scale was still relatively new at that date.
122. The next diagram from the Single Cell Oils book shows how the dried cells are treated, and the oil extracted, to produce crude DHASCO oil:
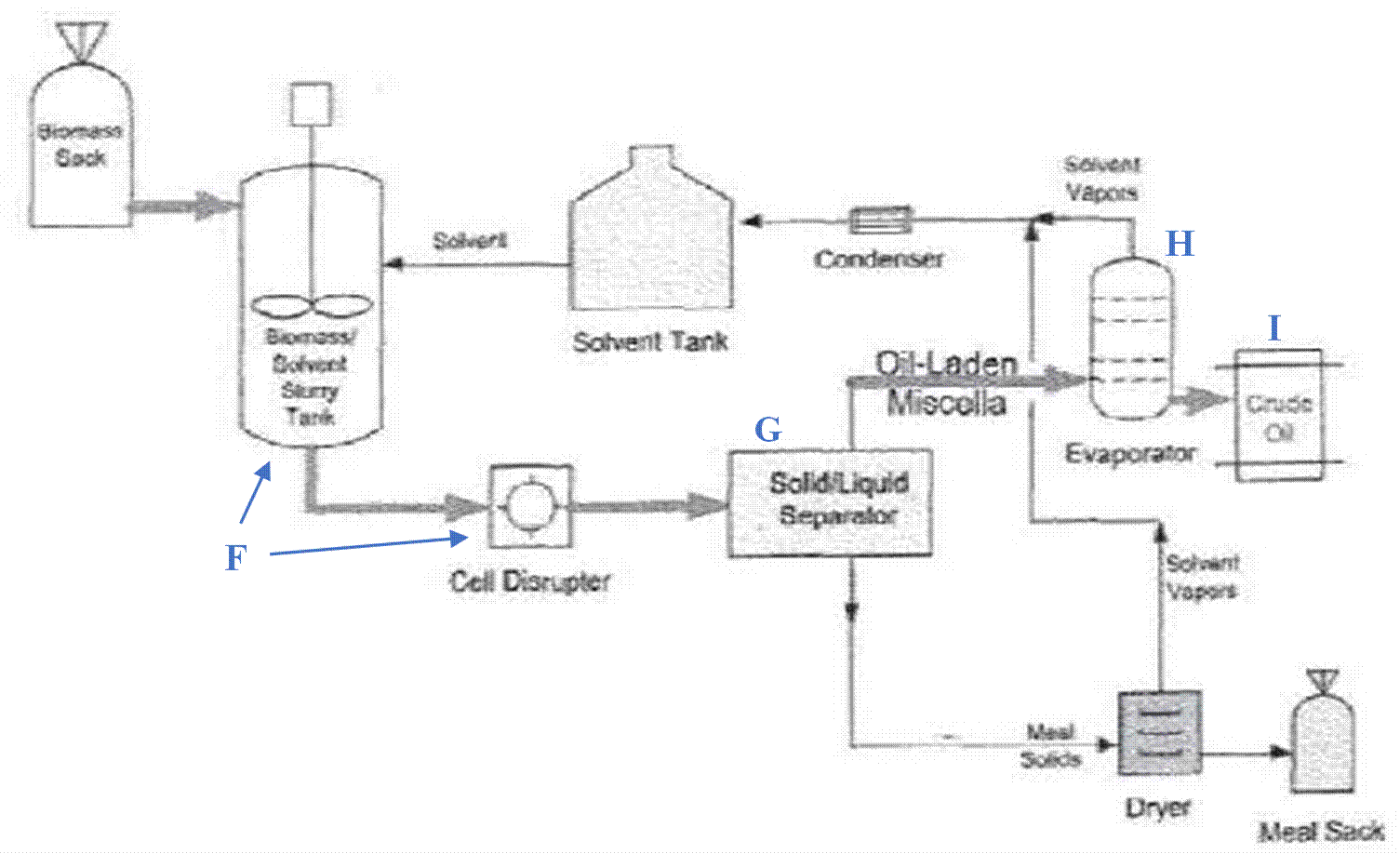
Figure 12: Overview of microbial oil production (to produce crude DHASCO oil)
123. As shown in step F, the next step in the production of DHASCO is to re-constitute the dried cells in a solvent (usually hexane) to make a slurry, and then use a ‘cell disrupter’ to lyse the cells (i.e. disrupting the cell walls to release the intracellular components).
124. A simple and effective means of disrupting cells is by the use of mechanical techniques. The two major mechanical techniques used to manufacture SCOs at the EP 155 Priority Date in May 2002 were:
i) High pressure homogenisation: This type of homogenisation involves subjecting the cells to very high pressure, and forcing the hexane-cell slurry through a narrow gap into a lower pressure environment, thereby creating shear forces which burst the cells open. The stream of cells could also be directed at a blade or plate where the high-speed collision aids in cell lysis. This was the method used in the process to manufacture DHASCO by Martek.
ii) Bead milling: (a.k.a. bead beating) This involves adding the hexane-cell slurry to a chamber containing beads (generally made of glass, ceramic or steel) and subjecting them to high-speed agitation, such as vortexing. The cells are physically ground against the beads, causing the cell walls to be disrupted and the intracellular components to be released. This technique is generally effective in lysing even very tough cells (and was commonly used to grind seeds to make seed oils), however it is harsher than other methods. This was the method used in the process to manufacture S-type DHA by OmegaTech.
125. Other lysis techniques at the EP 155 Priority Date in May 2002 were also known, but were not used in the commercial production of SCOs. Which techniques were or were not known is the subject of dispute, which I resolve later.
126. As mentioned above, as part of the extraction process for DHASCO the dry cells were first mixed into a slurry with an organic solvent prior to lysis. Lipids are insoluble in water, but soluble in suitable organic solvents. This means that lipid in the cells dissolves into the solvent, extracting the lipid from the dried biomass and leaving behind the other components, allowing separation. Hexane was the preferred and most commonly used solvent in industrial extraction processes in 2002. Hexane is a non-polar organic solvent well suited to dissolving non-polar lipids such as TAGs, which are released when the cells are disrupted. Again, this built upon the established practice of using hexane to extract oil from plant matter / seeds. The hexane-oil mixture, called the ‘miscella’, was then separated from the oil-depleted biomass, as shown in step G, using a centrifuge or decanter.
127. Finally, as shown in step H, the miscella (i.e. oil/hexane mixture) was passed on to an evaporator to remove the hexane (which is recovered and reused), leaving the crude oil as shown at I.
128. The FRIOLEX process was first developed in 1998, in the plant / seed oil context, as an alternative to traditional hexane-based downstream oil extraction. The FRIOLEX process starts with wet biomass following which the microbial cells are lysed in order to release the oil. The resulting water / lipid mixture (which takes the form of an emulsion) is treated with isopropanol (or a similar polar solvent) in water, agitated and then centrifuged. The water-miscible isopropanol makes the aqueous phase even more polar, and so less favoured by the non-polar lipids (such as TAGs). The small droplets of non-polar lipid therefore coalesce into larger lipid droplets, and the composition separates into a light oil phase and a heavy water/solvent phase, which can be separated using centrifugation. It was also known that salt could be added to increase the density of the heavy water/solvent phase and encourage better separation. The FRIOLEX process therefore offered a clear advantage, as hexane could be avoided. However, one disadvantage was that extractions tended to be less selective as compared to hexane. Moreover, the solvent needed to be recovered from the aqueous phase, which was challenging, as part of the point of the process was that the solvents were selected to be water-miscible.
129. The differences between traditional hexane extraction and the FRIOLEX process can be summarised as below:
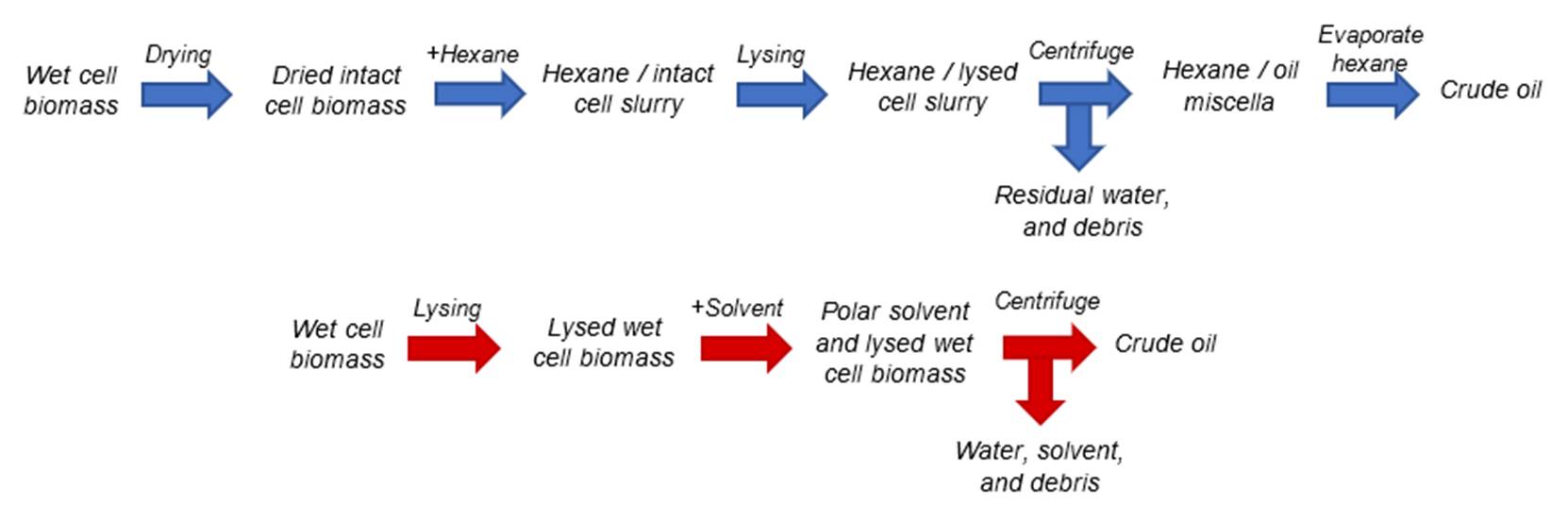
Figure 13: Outline comparison of hexane (top, blue) and FRIOLEX (bottom, red) methods
130. Although the Skilled Person/Team would have been aware of the FRIOLEX process in 2002, it had not at that point been implemented in a commercial scale microbial oil production process. Nevertheless there was increasing interest in the FRIOLEX process.
131. It had also long been appreciated that, in an ideal situation, the extraction process would be entirely aqueous. However, inherent in this was the likelihood that an emulsion forms between the lipid and water/other aqueous components. An emulsion is a mixture of two (or more) liquids that are normally immiscible, but where one liquid is present as microscopic droplets distributed throughout the other liquid(s). The more energetically stable the emulsion, the more difficult it will be to ‘break’ and separate the liquids. In standard solvent extraction, one reason that the biomass had to be dried (typically one of the more expensive steps in the production process) was that hexane and wet cells form an extremely stable emulsion under certain conditions that is very challenging to break.
132. Once oil had been extracted and processed, any organic solvent used in the extraction process needed to be removed. Thus:
i) Hexane was evaporated off (Dueppen XX [T2/104/4-12]).
ii) Any solvent used had to be removed so that it was only there in very small quantities, particularly if the lipid was to be used in a foodstuff (Dueppen XX [T2/166/21 - 167/4, 271/16 - 272/4]).
iii) This applied equally to a polar organic solvent in the FRIOLEX process - the oil at the end of the process would be understood to be substantially free of both water and organic solvent (Dueppen XX [T2/267/13-19]).
133. The result of the process described above is a crude oil, which may still contain some impurities. PUFA oils are very sensitive to degradation, and even relatively small amounts of certain impurities can lead to oxidation, discoloration, an unpleasant odour, or cloudiness at low temperatures.
134. The processing of microbial crude oils has followed the processes and principles applied to the refining of vegetable oils over the past 100 years. Crude microbial oils, like crude vegetable oils, are typically unfit for human consumption as they contain numerous impurities and often have an odour and unacceptable taste. Impurities which are removed include water, free fatty acids, phospholipids, minerals, carotenoids, sterols, antioxidants, waxes and residual cell debris. Since microbial oils are sensitive to oxidation, care needs to be taken over these techniques. In contrast to crude oils produced from fish, crude microbial oils are free from pesticides, insecticides, heavy metals and other pollutants often found in fish oils. Nevertheless, downstream processing would have been undertaken as a matter of course for oils which were to be incorporated into food products or any other products for human consumption.
135. A trio of steps called refining, bleaching and deodorisation form the standard process, which is commonly abbreviated to RBD. These techniques are essentially identical to those used for many decades in the plant / seed oil industry, which had been developed, automated, and made into a continuous process by the 1950s.
136. Refining involves both degumming (a water washing process which removes phospholipids by hydrating them to form a sludge / gum) and neutralisation (fatty acids are neutralised by the addition of sodium hydroxide making them more soluble in water, so again can be separated from the oil with a water wash). The purpose of bleaching is to improve the colour of the oil by removing the remaining impurities including pigments, trace metals, and oxidation products. Deodorisation, the last step in the overall refining process, aims to remove odiferous small molecules.
137. The oil may also undergo winterisation, which is sometimes considered a separate precursor to RBD, but sometimes considered a refinement step itself (and the acronym RBWD is sometimes used). Winterisation is a type of fractional crystallisation involving cooling the oil, so that the components with higher melting points (e.g. saturated fatty acids) solidify (crystallise). The precipitated components can then be separated from the liquid (e.g. via filtration). The aim is to achieve an oil which remains clear and does not turn cloudy at low temperatures.
138. The result of these steps is a refined oil, which can be incorporated into various products.
139. As the process to make a microbial oil is a biological one, the percentage of the relevant fatty acid obtained (e.g. DHA) would vary in each production run by a few percent. Therefore, it was also common to blend different batches of a refined oil together, and also to dilute refined oil with a small amount of another oil (e.g. sunflower oil), to make a standardised product. For example, in the case of DHASCO, after refining, bleaching, winterisation and deodorising, tocopherols and antioxidants were added to the oil to prevent any further oxidation, following which it was blended with high-oleic sunflower oil to a standardised 40% DHA for commercial sale.
140. PUFAs are generally highly susceptible to oxidation. Oxidation causes the lipids to change in flavour, odour and also colour, in turn causing the oil to become rancid, limiting its shelf-life, and causing a ‘fishy’ smell and taste. Oxidation is a risk throughout the production process and during storage - it can be caused, or accelerated, by exposure of the PUFAs to oxygen, heat, light, oxidative enzymes, certain metals etc. As such, processes were designed to minimise the extent of oxidation. Common steps taken included de-oxygenating using inert gases such as nitrogen, pasteurising to deactivate endogenous enzymes, and adding chemical antioxidants to the oil when storing.
141. There are two very common analytical measures of PUFA oxidation which each provide a simple numerical value, in both cases a lower value indicating less oxidation:
i) Peroxide value (PV) is a measure of the amount of peroxide present in a lipid sample. The primary oxidation of lipids predominantly causes the formation of hydroperoxides, which are captured in the PV, and so PV is a measure of the components produced in the early stages of oxidation. As such it provides a ‘snap shot’ of the oxidation status of a sample at a single point in time.
ii) Anisidine value (AV) (also referred to ρ-AV or AnV) is a measure of secondary oxidation of a lipid sample. As it can capture the products of oxidation occurring over a period of time, AV is considered to be a useful measure of the ‘oxidative history’ of a sample.
142. Bill Barclay was a prominent figure in the field of microbial oils, and both Dr Wynn and Mr Dueppen were previously aware of the work reported in the Barclay prior art (Wynn 1 ¶111; Dueppen 1 ¶79). Dr Barclay’s company was OmegaTech, and OmegaTech patents were a source of information about thraustochytrids including Schizochytrium (Wynn 2 ¶16).
143. Barclay had identified a Schizochytrium strain that was deposited as ATCC 20888. It was CGK in 2002 that this was the source of OmegaTech’s commercial DHA oil, known inter alia as S-type DHA (Wynn 1 ¶¶102-103, 276; Agreed CGK - see above). While Martek’s DHASCO and ARASCO dominated the market, S-type DHA had a notable part of the total sales of microbial oils in the period up to 2003 (Figure 10 at Wynn 1 ¶101).
144. Both Dr Wynn and Mr Dueppen agreed that Schizochytrium strain ATCC 20888 was well known in 2002 as a good source of DHA (Dueppen XX [T2/100/3 - 101/9, 148/16-19]; Wynn XX [T4/447/25 - 449/4]).
145. Mr Dueppen exhibited Chapter 3 of the first edition of Single Cell Oils as describing OmegaTech’s CGK process in 2002 for producing S-type DHA from this strain (Dueppen 1 ¶58; [C4/3]). Chapter 13 of the first edition of Single Cell Oils also refers to this use of Schizochytrium ([D2/4/56]; Dueppen XX [T2/122/4 - 123/24]).
146. Going into the trial, DSM maintained that certain advantages associated with Barclay’s Schizochytrium strain (as set out at paragraph 88 of the Statement of Agreed CGK [B1/35/305]) were not CGK in 2002, but had become so by 2009 - this was point 5 in the List of Disputed Issues. Mara’s position was that this information was already CGK in 2002, and this was confirmed by Dr Wynn [T4/450/12 - 452/16].
147. These advantages, as compared to the production of DHASCO from C.cohnii, were:
i) The Schizochytrium strain grew at low salinity - an advantage for growth in standard stainless steel tanks. See Wynn XX [T4/451/23 - 452/3].
ii) Low dissolved oxygen levels had been shown to induce thraustochytrid DHA production. This meant that the Schizochytrium strain used to produce S-type DHA could be grown in larger culture vessels without a concern for depletion of oxygen in different parts of the tank. This was in contrast to C.cohnii, which required oxygen to produce DHA. See Wynn XX [T4/452/8-12].
iii) The Schizochytrium strain grew to higher cell densities at a faster rate than C.cohnii. See Wynn XX [T4/450/21 - 451/22].
CGK Disputes regarding 3 May 2002
148. Here I set out and decide the remaining points in dispute on the CGK.
149. The parties managed to disagree even over the formulation of this dispute. DSM characterised the issue as ‘Whether the particular composition and structure of the cell wall of Schizochytrium cells (or Thraustochytrids more generally) was CGK or whether it was something that the Skilled Person would have found out.’ (emphasis added). The dispute arose in the context of enzymatic lysis. As phrased, the issue is not a CGK issue at all - what the Skilled Team would find out is best considered in the context of obviousness. However, the first part of that issue is too limited. The issue is best characterised as ‘What, if anything, was CGK concerning the composition and structure of the cell wall of Schizochytrium cells (or Thraustochytrids more generally)’.
150. Where all the evidence ended up was, in my judgment, as follows. The Skilled Team would not know the particular composition or structure of the cell wall of Schizochytrium cells (as reported in the Darley paper from 1973), but they would know in general that microbial cells had a cell wall and a cell membrane and that the three main components were carbohydrate, protein and lipid, as Mr Dueppen accepted in cross-examination. Dr Wynn accepted in cross-examination that the Skilled Team would know that protein would make up at least in the order of 15-22% of Schizochytrium cell walls.
151. Furthermore, that the Skilled Team would have some knowledge of (or would look up) cell wall structure and composition of a microorganism of interest is confirmed by this extract in Chapter 13 of the SCO Book, in a section entitled ‘Extraction of DHA-rich oils from Crypthecodinium cohnii and Schizochytrium sp.’ Under the heading ‘Pre-Treatment and Cell Disruption’ the authors state:
‘Both microorganisms considered in this section are protected by extremely tough cell walls. In order to release their cellular contents, a number of methods for cell disintegration have been developed. These methods fall into three major categories: chemical, biological and physical. Some of the methods have severe limitations with regard to large-scale application, compatibility with the product, or cost.
Knowledge of cell wall structure and composition is, therefore, important to optimize chemical methods and achieve cell lysis without damage to the DHA oils. For mechanical methods, size, shape and degree of cross-linking of structural polymers are important factors to determine the ease of disruption. Nevertheless, mechanical methods, especially wet milling in high-speed agitator bead mills and high-pressure homogenizers, have demonstrated good performance on a large scale for cell disruption of microorganisms with tough cell walls, including microalgae. It is desirable to achieve as complete cell disruption as possible through the optimisation of processing variables including flow-rate, pressure, temperature and disruption chamber design and operation. Some of the variables involved in cell disruption have been reviewed (ref 13). The disintegration process, therefore, will strongly influence the solid-liquid separation in the downstream processing and the overall extraction yield.
The ease of cell disruption is also related to fermentation growth conditions. Fast growth rates, in general, produce cells having weaker cell walls since they do not have time to produce material to reinforce the cell wall structures. Schizochytrium sp. a faster growing algae than C. cohnii, possesses an intrinsically weaker cell wall and, as a consequence, energy requirements for its disruption are significantly lower.’
152. This passage indicates that the situation is not nearly as simple as ‘you only need to know about cell wall composition and structure for enzymatic lysis’. Whilst someone in the industry could simply apply mechanical lysis, this passage indicates that the Skilled Person would investigate those features precisely because that knowledge would secure a better process, results and yields.
153. Beyond that, if the Skilled Team was interested in enzymatic lysis of Schizochytrium cells there were two ways in which they might find out further information. They might be motivated to undertake a literature search which would reveal the Darley 1973 paper which gives details of the cell wall composition. However, in my judgment, what they would try first was a simple and routine empirical test in the lab. Grow some Schizochytrium cells and apply a non-specific alkaline protease. Then analyse the product using a microscope to gauge the degree to which cell lysis had occurred. They might also use a carbohydrase in addition, but either way they would find from their lab experiments that enzymatic lysis would work on Schizochytrium cells.
154. This was an important area of dispute. Two ‘brute force’ methods - see [124] above - were used in commercial production. The principal dispute was whether and to what extent enzymatic lysis was a CGK technique.
155. In closing, DSM contended it was common ground that the skilled person would have no reason to look for a new way of lysing and no conception of enzymatic lysing being relevant to their work in the microbial oils field. This submission was based in part on this passage of cross-examination of Dr Kyle:
20 Q. Can I suggest this, now we have looked at that, thinking about
21 the common general knowledge, as reflected in these documents.
22 A. Okay.
23 Q. I think we can all agree they show that the skilled person
24 would be aware of enzymatic lysis as a concept; yes?
25 A. Yes.
2 Q. No doubt they did it at university or probably high school;
3 yes?
4 A. Accurate; yes.
5 Q. They would be aware that enzymatic lysis had certain
6 applications, in particular where selectivity is required;
7 yes?
8 A. Yes.
9 Q. But they would have no conception of it being relevant to
10 their work with microalgae or the extraction of lipids from
11 microalgae?
12 A. Unless they were looking for a new way of lysing that is a
13 more gentler form than hitting it with a hammer, then they
14 would be interested in it.
15 Q. Subject to that, the answer to my question is "Yes"?
16 A. That was a, "Yes, but".
156. Thus, DSM appeared to develop a mindset argument against any lysis technique other than homogenisation or bead milling. These contentions were based on the written evidence from Mr Dueppen and Dr Wynn.
157. However, in his first report, Mr Dueppen had addressed the Barclay Patent, later dropped as prior art against EP155, in which he noted that
‘“enzymatic digestion of the cell wall” is in the list of “well-known” lysis techniques. However, I do not believe that the Skilled Bioprocessing Engineer would have considered enzymatic digestion as well-known at all. When I first read Barclay, I did not place any significance on this sentence..’
and
‘…although it is a matter of basic science that different enzymes break down different substances, including those which may be found in the walls of certain cells, at the EP 155 Priority Date it was simply not something that the Skilled Bioprocessing Engineer would have given any thought to.’
158. When discussing the teaching in [0027] of EP155, Mr Dueppen accepted that the SBE would have been aware that:
i) Different microorganisms have different cell walls (e.g. composition and structure).
ii) Different classes of enzymes exist, and any enzyme would need to be suitable for the microorganism in question.
iii) Each enzyme has an ‘activity profile’, and in particular a range of pH and temperature values where it shows good activity. Outside of those conditions activity drops and the enzyme can begin to denature and/or become inactive.
159. Particularly for the first two points above, he said the detailed knowledge and experience would lie with the upstream team. In particular, microorganism selection is led by the upstream team, and the Skilled Bioprocessing Engineer would tend to rely on the Skilled Microbiologist to assess the structure of the cell wall of a particular microorganism, and so also the choice of enzyme to use.
160. I am very well aware that Mr Dueppen gave that evidence in the context of EP155, but his three sub-paragraphs provide at least some indication of the level of CGK on enzymatic lysis.
161. In his second report, Mr Dueppen responded to a review article cited by Dr Kyle at his DJK-7 entitled ‘Pilot- and Process-Scale Techniques for Cell Disruption’ dating from 1990, by saying:
‘The paper …. is a general discussion of a host of lysis techniques (with sections on physical/mechanical, chemical and biological methods including enzymatic lysis) which could potentially be deployed in a number of contexts. The abstract notes that physical/mechanical methods are mostly “universally suited”, while a chemical and biological method “offers improved selectivity” but “requires individual procedures for each product”. Again, microbial oil production was not a process that required selectivity when it came to lysis, and the mindset of the Skilled Bioprocessing Engineer is important in this respect. If the Skilled Bioprocessing Engineer was asked a general question whether enzymes could lyse cells, I think they would say that it could probably be done, but they would have to investigate which enzyme(s) might lyse which particular cell(s) and under which conditions, and for that purpose would seek assistance from the Skilled Microbiologist. However, that was not the mindset of those in the field of microbial oil production at the time, and there was simply no need to consider the question in the first place, as the field was focused on using simple physical/mechanical methods that had been successfully used for years.’
162. Dr Wynn’s evidence was to similar effect. In his second report, he addressed Dr Kyle first report on this topic:
‘19. Kyle 1 paragraph 113 discusses biological methods to break open cells. I agree that the Skilled Microbiologist would have known that biological methods could be used to break open the cells of certain types of microorganisms and that this method had been applied in certain contexts. However, SCO production was not one of those contexts.
20. As noted by Dr Kyle in paragraph 114 and supported by the article provided as Exhibit DJK-7, some knowledge of the cell wall structure and composition was important for developing biological (and chemical) methods of cell lysis. As I explain further below, this meant that biological methods were only considered by scientists when there was a specific need that warranted the research to identify the correct enzyme (or enzyme combinations) that would work to break open the cell wall of the microorganism. However, this need had not arisen in microbial oil production, where simple techniques such as mechanical lysis had been established, scaled up and economically deployed.
23 …DHA could be extracted from Crypthecodinium and Schizochytrium cells using mechanical methods to produce a high-quality oil. Further, whilst there are capital costs associated with physical methods, enzymatic methods themselves involved significant cost (e.g. sourcing the enzyme itself). At the EP 155 Priority Date, the Skilled Microbiologist would not have been motivated to explore enzymatic methods of extracting lipids from microbial cells.
29. Therefore, regardless of whether the biological methods of breaking open cells discussed by Dr Kyle would have been familiar to the Skilled Microbiologist in a general sense:
1) Enzymatic methods had not been used to in the processes for single cell oil extraction and there was no motivation to do this at the EP 155 Priority Date; and
2) The application of enzymatic methods to break open one type of microbial cell does not assist the Skilled Microbiologist in understanding what type of enzyme (or combination of enzymes) could be used to break open a different type of microorganism without knowledge of its cell wall structure and composition.’
163. The cross-examination revealed a somewhat different picture. Whilst both Dr Wynn and Mr Dueppen made it clear that the brute force methods (homogenisation and bead milling) were used in commercial production, Mr Dueppen accepted that the Skilled Team knew that there were other methods (this being one of a number of similar passages in his evidence):
19 Q. You did know there were other methods?
20 A. Particularly useful for small-scale, bench-top, but as a
21 production and bioprocess development engineer, we were
22 looking for ways to scale these up to do large volumes of
23 processing.
164. Dr Kyle’s evidence was that enzymatic lysis was CGK and he referred to a passage in the Single Cell Oils book, chapter 13 in support. That enzymatic lysis was CGK is reflected in a number of documents:
i) First, see the quote from the SCO Book in [151] above. Mr Dueppen agreed that ‘biological’ in that quote refers to the use of enzymes. Mr Dueppen also agreed that the skilled person knew about enzymatic lysis as one of the three major classes of lysis processes.
ii) The Barclay prior art, filed in 1990 and published in 1992, treats enzymatic approaches as a CGK technique: it says that “harvested cells (fresh or dried) can be ruptured or permeabilized by well-known techniques such as … enzymatic digestion of the cell wall” (col.13, l.43-49). Barclay’s work was well known in the field.
iii) The Bijl prior art, filed in 2000 and published in 2002, treats it similarly - it refers to “disrupting or lysing the cell walls of the microbial cells, for example by a physical, enzymatic or mechanical technique” ([B3/2/34] col.5, l.44-46; see [189] below).
165. Mr Dueppen agreed that the references in Single Cell Oils, Barclay and Bijl reflected CGK of enzymatic lysis, and that even his downstream skilled person was aware that it could work.
166. So, although enzymatic lysis was CGK, it was another matter as to how the Skilled Team would approach a suggestion to use it. That depends on the context.
167. The other disputes over lysis concerned the extent to which:
i) centrifugation; [Kyle 1 ¶103; Dueppen 2 ¶21]
ii) spray drying; [Kyle 1 ¶¶101,110; Dueppen 2 ¶27] or
iii) isopropanol [Kyle 1 ¶¶103, 106, 195; Kyle 2 ¶26; Dueppen ¶¶22-26; Wynn 2 ¶35] DSM closing 61-63
could be expected, or intended, to cause or contribute to cell lysis.
168. It was agreed that it was possible that both centrifugation and spray drying could result in a degree of lysis, but Mr Dueppen gave unchallenged evidence that:
i) In the case of centrifugation, the effect would be minimal, particularly in the context of microbial oil production where cells were relatively tough to lyse.
ii) Spray drying was actively to be avoided because if cells ruptured during spray drying the released oil could violently combust.
169. The dispute over isopropanol causing lysis arose because of Dr Kyle’s attempt to argue that the Skilled Team would understand that it was isopropanol which caused the lysis in EP155 Example 3. Mr Dueppen said this was wrong and, by closing, the contention that isopropanol caused the lysing had been abandoned.
170. For completeness I mention one further CGK dispute which was raised by some evidence in Dr Kyle’s reports: whether it was CGK that damaging the cell wall using a lysis technique could be insufficient to release the oil bodies because (i) the cell membrane could remain intact; or (ii) a ‘skeleton’ of the cell wall could remain intact. This dispute over the formation of protoplasts during lysis was abandoned following Dr Kyle’s cross-examination. I agree with DSM that it was not a relevant consideration for the Skilled Team.
171. Although it is necessary to consider the effect of DSM’s mindset argument later when considering the arguments on obviousness, it may be helpful to outline the common theme, which will already have appeared from what I discussed above.
172. DSM contended that enzymatic lysis was not CGK because it was not considered ‘a good basis for further action’. It was clear to me that that contention was based on the viewpoint of those involved in large scale commercial production. Furthermore, it was tolerably clear to me that when Dr Kyle gave his answer in the passage of cross-examination quoted in [155] above, he gave it in the context of commercial production. To my understanding, the mindset argument was effectively that mechanical lysis worked in commercial production and there was no incentive to look for or examine other forms of lysis.
173. It was clear that forms of lysis other than mechanical were available and known and may well have been the subject of small-scale experiments in the lab or academia. However, I received very little evidence of what was actually occurring in the lab or in academia.
174. In terms of disincentives in the commercial world against the development beyond mechanical lysis and the existing established production methods, or, indeed, any new business in this field, three other points were identified in the evidence by way of commercial barriers:
i) First, Martek’s patent position.
ii) Second, Martek’s commercial dominance.
iii) Third, the need for regulatory approval for any new source of PUFA, a process which required significant investment.
EP155
175. By the time of closing argument, the only issue on EP155 concerned inventive step over Bijl, Mara having dropped their insufficiency challenge. It is convenient to consider the disclosure of Bijl before that of EP155.
176. There were major disputes over three aspects of Bijl:
i) First, a dispute over the ‘focus’ of Bijl.
ii) Second, how the Skilled Team approaches Bijl.
iii) Third, what the Skilled Team would do, having read Bijl (in the usual way, with interest).
177. These disputes are all interrelated and all depend, in one way or another, on what Bijl actually says.
178. Bijl is a European Patent application published in February 2002. It happens to be the priority document for the “Hendrik” prior art and therefore the evidence going to Hendrik was to a large extent equally relevant to the case on Bijl (especially the evidence as to the skilled person’s reaction to Hendrik and what they would do with it).
179. I approach Bijl without any preconceptions, but with the CGK and the attributes of the Skilled Team in mind. In view of the rival submissions as to the focus of Bijl, it is necessary to pay attention to the opening paragraphs which set the scene for the more detailed disclosure.
180. Bijl is entitled ‘Isolation of microbial oils’. The specification begins with [0001] stating:
‘The present invention relates to the extraction (and then isolation) of a microbial (or single cell) oil, preferably comprising one or more polyunsaturated fatty acids (PUFAs), from single cell (or micro-) organisms. The process of the invention involves the disruption or lysis of microbial cell walls, followed by separating the oil from the resulting cell debris. The invention additionally relates to a microbial oil recovered by this process, preferably having a PUFA.’
181. In context, it is clear that Bijl is here using ‘extraction’ to cover lysis and ‘isolation’ to cover the recovery of the microbial oil.
182. [0002] describes how PUFAs are found in a wide variety of organisms and have many uses. [0003] continues:
‘[0003] In most microbial PUFA production processes a microorganism is first cultured in a fermenter in a suitable medium. The microbial biomass is then harvested and treated to enable subsequent extraction of a lipid from the biomass with a suitable solvent. The lipid is usually subjected to several refining steps. Care must be taken during the process because degradation can occur if the lipids are subjected to lipolysis or oxidising conditions, for example heating (in the presence of oxygen) and/or due to lipases or lipoxygenases. The art teaches that to avoid oxidation (such as resulting from breaking open the cells and so exposing the contents to oxygen) PUFAs can be extracted from whole intact cells using a solvent (see WO-A-97/36996 and WO-A- 97/37032). The use of solvents is a common way of removing lipids from microbial biomass (WO-A-98/50574).’
183. Bijl then continues to explain the problems with that approach at [0004], where ‘extraction’ is now being used to cover the recovery of the microbial oil:
‘Although these extraction processes [i.e. using a solvent] have been used for several years, the solvent needs to be removed and this results in extra cost. In addition, if the lipid is to be used in a foodstuff, it is important that certain solvents, such as hexane, are removed completely, or only remain in very small quantities. If the hexane is removed by evaporation then this may involve heating and that not only adds to costs but can cause lipid degradation. Furthermore, with increasing environmental considerations, the use of solvents for the extraction of lipids is becoming increasingly expensive and unpopular.’
184. At [0005], Bijl says
‘The present invention therefore seeks to solve or at least mitigate these problems. The Applicant has found that lipids; such as those comprising a PUFA, can be efficiently extracted from microbial cells without the need for solvent(s).’
185. In [0006] Bijl explains a ‘first aspect’ of the invention, being
‘…a process for obtaining an oil (or fat or lipid, the terms are used interchangeably) from microbial cells, the process comprising (a) disrupting (or lysing) the cell walls of the microbial cells to release the oil from the cells. The (microbial or single cell) oil can then be (b) separated from at least part of the resulting cell wall debris. One can then (c) recover, purify or isolate the microbial oil (or one or more PUFAs). A good yield of the oil can be achieved using this process without the need for a solvent. Preferably the oil will comprise one or more PUFAs.’
186. In [0007], Bijl addresses a suggestion in one piece of prior art:
‘[0007] Recent PUFA preparation processes advocate keeping the microbial cells intact (WO-A-97/36996). The PUFA is then extracted from the intact cells inside the granules by contact with a solvent, usually hexane. The hexane is then evaporated to produce a crude oil. Throughout this process the cells are kept intact to prevent oxygen in the atmosphere contacting the PUFAs and causing undesirable oxidation. However, it has now been found that a good quality PUFA oil can be achieved if the cells are in fact lysed: any potential oxidation by the atmosphere is more than compensated by the advantage of avoiding the need for solvents.’
187. Under the heading ‘PUFAs and microorganisms’, Bijl then discusses suitable organisms, noting at [0008] that the microbial cells may be bacteria, yeast, algae or fungi. This paragraph lists genera of preferred fungi including Mortierella and Thraustochytrium, which were both well known as sources of microbial oils, in particular those containing PUFAs, although the skilled person would know that by 2002 Thraustochytrium was classified as an algae. Among algae the well-known source of DHASCO, Crypthecodinium cohnii, is listed at [0009].
188. At [0010] preferred PUFAs are given, which include DHA, ARA and EPA, and in [0011] possible sources of these are listed, including the familiar Mortierella for ARA and Crypthecodinium or Thraustochytrium for DHA.
189. Bijl describes at [0013] how, after fermentation (discussed in [0012]), the wet biomass can be separated from the culture medium by centrifugation or filtration.
190. The next section is entitled “Cell lysis” and deals with how to lyse the biomass. It begins with [0014]-[0015]:
‘[0014] The cell walls of the microbial cells can then be disrupted (or lysed). This can be achieved using one or more enzymatic, physical or mechanical methods or techniques, for example at high shear conditions. Physical techniques include heating and/or drying the cells to a sufficient temperature whereby the cell walls are ruptured. This may comprise boiling.
[0015] Enzymatic methods include lysis by one or more enzymes, e.g. cell wall degrading enzymes. The cell wall degrading enzyme may be a lytic enzyme. Other enzymes include (e.g. alkaline) proteases, cellulases, hemicellulases, chitinases and/or pectinases. Other cell wall degrading substances may be used instead of or in combination with one or more enzymes, e.g. salts, alkali, and/or one or more surfactants or detergents. A combination of physical, mechanical and/or enzymatic methods is also contemplated.’
191. As Dr Kyle noted in his first report, these were all CGK techniques. However, it is clear that Bijl plainly proposes the use of cell wall degrading enzymes for lysis (including proteases), and the combination of an enzyme and surfactant.
192. [0016] discusses and gives details of mechanical techniques, but [0017] states that homogenization is the preferred method of disrupting the cell walls and gives details of the pressures that can be employed in, for example, a Gaulin homogenizer.
193. [0018] says that chemical lysis is preferably not employed, as the process is preferably solvent-free.
194. Mara were keen to submit that Bijl does not denigrate enzymatic lysis and drew attention to claim 2, where “the cells are physically, enzymatically or mechanically disrupted, optionally by homogenisation”.
195. The section on ‘Separation’ starts at [0021]. Bijl prefers the mechanical method of centrifugation as a way of separating the oil from the cell debris:
‘[0021] The microbial oil is then separated from at least part of the cell wall debris formed. At this stage the PUFA may be in an oily or lipid layer. This may be a top or upper layer, which is (or has risen) above an aqueous layer containing cell wall debris. The oily layer comprising the PUFA can then be separated from the aqueous phase. One or more surfactants or detergents may be present or added to assist this process.
[0022] The separation of the oil from at least some of the cell wall debris is preferably achieved or assisted by using a mechanical method, in particular by centrifugation.’
196. [0022] goes on to list suitable centrifuges, for both industrial and lab scale work, the maximum centrifugal force they exert and suitable flow rates, and concludes:
‘Centrifugation may result in either a 2-phase system (a fatty or oily top layer and a lower aqueous layer) or a 3-phase system (a fatty or oily top layer, a middle aqueous layer and a bottom layer, usually containing the cell debris).’
197. At [0024] Bijl emphasises the solvent-free nature of the lysis and separation steps (in this context, ‘solvent’ excludes water).
198. [0025] also says that the use of a surfactant is preferably avoided. Again, Mara were keen to point out that Bijl does not ignore this as an option. To the contrary, it claims it in claim 9, together with (or instead of) use of cell wall degrading enzymes:
‘9. A process according to any preceding claims wherein the disruption of the cell walls is assisted by one or more cell wall degrading enzymes or surfactants.’
199. Under the next heading of ‘Overall Protocol’, [0028] sets out a preferred process. The lysis step is described as follows:
‘(e) disrupting or lysing the cell walls of the microbial cells, for example by a physical, enzymatic or mechanical technique (such as homogenisation, e.g. with an homogeniser or a ball mill). This releases some of the oil and/or PUFA present in the microbial cells. The (mechanical) disruption may be supplemented with or substituted by chemical and/or enzymatic disruption.’
200. As Mara pointed out, the options in this preferred process are physical, enzymatic or mechanical techniques. However,
‘The (mechanical) disruption may be supplemented with or substituted by chemical and/or enzymatic disruption”.
201. The separation step is described in (f):
‘(f) separation of the microbial oil (or PUFA) from the cell wall debris, for example separation of the oil phase from the resultant cell wall debris and/or aqueous phase. This may comprise centrifugation, optionally with the addition of one or more salts, a pH shift (towards alkaline), and may involve the presence of one or more cell degrading enzymes, surfactants or emulsifiers;’
202. DSM characterised the list of separation possibilities (in their own patent application) as moving from the sublime (centrifugation alone) to the ridiculous (emulsifiers to break an emulsion). Although none of the experts made this suggestion, Counsel for Mara suggested that the reference to ‘emulsifiers’ is a typographical error and it should read ‘de-emulsifiers’. Although DSM poured scorn on centrifugation alone as a means of separation, the Skilled Team would note that centrifugation (alone) was the means of separation in both of the examples.
203. In common with the lysis step, Bijl says that separation avoids the use of a solvent, as does preferably the subsequent step of extraction and purification, making the process essentially solvent-free ([0024]).
204. The separation step is preferably assisted by centrifugation, with the possible addition of surfactants or detergents ([0021]-[0022]). [0028] also mentions optional addition of salts, use of a pH shift (towards alkaline), and presence of cell degrading enzymes. Dr Kyle’s evidence was that some of these methods were recognised as being able to break an emulsion (Kyle 1 ¶156; Kyle 3 ¶35), and it is common ground that at least salt was known to aid in breaking emulsions (Dueppen 2 ¶34), as was centrifugation (Dueppen 2 ¶37, although he says this depends on the strength of the emulsion). Thus, to the extent that an emulsion forms upon lysis, Bijl set out ways of dealing with it.
205. The subsequent downstream processing follows standard techniques (Kyle 1 ¶157).
206. Bijl contains two examples of solvent-free extraction. One uses Mortierella to produce ARA, and the other Crypthecodinium to produce DHA (i.e. the strains used by Martek to produce ARASCO and DHASCO, respectively). Both examples use homogenisation as the means of lysis, followed by centrifugation to generate an oily top layer and a lower aqueous layer containing cell debris.
207. In more detail, Example 1 in Bijl is ‘Preparation of crude PUFA (ARA) oil from a fermentation broth of Mortierella alpina.’ A pasteurized fermentation broth is subjected to homogenisation at 600 bar and then centrifuged at 8,000 rpm, equivalent to 8,000g at the disc stack,
‘resulting in an arachidonic acid-enriched oily top layer (that was recovered from the centrifuge) and a lower aqueous layer containing the cell debris. A crude PUFA oil was recovered: the yield of oil was 95% (based on the oil in the cell). The crude oil had the following approximate composition: 1 to 2% sterols and cell debris; 3 to 4% phospholipids; 4% monoglycerides; 6% diglycerides; and the remainder being triglycerides.’
208. Similarly, Example 2 is ‘Preparation of crude PUFA (DHA) oil from a fermentation broth of Crypthecodinium cohnii’. Lysis was carried out by homogenisation three times at 600 bar. Crude oil was recovered using a lab-scale centrifuge on 800ml portions.
‘This resulted in a DHA-enriched fatty top layer (crude oil) and a lower aqueous layer. A crude PUFA oil was recovered from the fatty top layer.’
209. Before I leave the topic of what Bijl discloses, there are two particular sub-topics I should address. The first concerns a dispute between the parties over the ‘focus’ of Bijl.
The dispute over the ‘focus’ of Bijl
210. DSM were keen to stress that the focus of Bijl is lipid separation without the use of solvents, and therefore it would be of primary interest to the Skilled Bioprocessing Engineer member of the team. In closing, Mara disagreed with this characterisation, suggesting that is not how the document presents itself. However, in their opening, Mara had presented Bijl as relating to “a solventless extraction process for production of microbial oils, which the experts agree would be of interest”. In closing, Mara suggested that what Bijl discloses as its invention is the very use of cell lysis in the extraction of microbial oils, claiming that the suggestion that the process is preferably done without use of solvent is a consequence of lysing, not the focus of the document.
211. In my view, the Skilled Team reading Bijl does not need to form a view as to the ‘focus’ of the document. Instead, they would just consider its teaching. That said, the arguments as to ‘focus’ do have some significance when considering what the Skilled Team would do having read Bijl.
DSM’s list of the ‘problems’ with Bijl
212. DSM were keen to highlight a number of ‘problems’ with Bijl, suggesting that the Skilled Team would not regard Bijl as a serious and reliable technical document. I have adverted to some of them already:
i) First, DSM characterised [0003] as suggesting that production of microbial oil was normally done without lysis. This misreads the document. All Bijl says, by reference to two prior art patent applications, is that ‘PUFAs can be extracted from whole intact cells using a solvent’ and one of those applications is referred to again in [0007] where it says ‘Recent PUFA preparation processes advocate keeping the microbial cells intact’. The SCO Book also refers to such processes, the implication being that they were actually in use, as opposed to being mere suggestions in patents or applications.
ii) Second, the ‘out of date classification’ of Thraustochytrium as a fungus. DSM’s point is correct.
iii) Third, the failure to acknowledge that an emulsion would form or properly address how to break it. DSM submitted that this omission was particularly striking because of their view that separation of the oil is actually what Bijl is about.
iv) Fourth, what DSM characterised as the odd suggestion at [0028](f) of using a pH shift to separate the oil. DSM suggested the Skilled Team would not accept this.
v) Fifth, what DSM characterised as the absurd suggestion of using an emulsifier to separate the oil.
vi) Sixth, the Examples which appear to claim that centrifugation alone would break the emulsion caused by mechanical lysis.
vii) Seventh, DSM pointed to the breadth of claim 1 as an attempt to claim what the Skilled Team has been doing for years. I am inclined to discount this point on the basis that the patentee was no doubt seeking the widest possible protection and had a series of fall-back positions in the later claims.
213. To the extent necessary, I have taken account of these points and will do so when I turn to consider what the Skilled Team would do, having read and considered Bijl.
214. EP155 was filed on 5 May 2003, so has expired. It has an uncontested priority date of 3 May 2002. The patent is entitled “Method for producing high-quality lipids by enzymatic liberation from biomass”. [0001] adds the detail that the invention is about using microorganisms of the genus Schizochytrium.
215. EP155 describes a method for producing high-quality lipids from Schizochytrium microorganisms using an alternative lysis method which does not have the downsides of known techniques.
216. The downsides of existing methods of extracting lipids from biomass are described at [0003]:
‘Problems with prior methods include poor product quality due to chemically aggressive conditions of high temperature and high pH, high costs due to the need to dry the biomass or for the additional equipment such as homogenizers and pressure vessels.’
217. The following paragraphs set out issues which can arise with PUFAs as a result of the oxidation of their double bonds, and refer to some prior art, including both pieces of prior art relied on by Mara (Barclay and Bijl).
218. The Summary of the Invention is at [0012] to [0015], setting out that the invention provides a method that comprises contacting (i.e. treating) the Schizochytrium biomass, which contains a PUFA, with a protease enzyme and then recovering the lipid. The resulting lipid comprising the PUFA is stated to have a low anisidine value. The PUFAs in the lipid can be DHA, docosapentaenoic acid (“DPA”) or arachidonic acid (“ARA”) and [0014] notes that these PUFAs can be incorporated into various products including infant formula.
219. As Dr Kyle explained, the skilled person would have recognised that DHA, DPA and ARA were PUFAs produced in work done by the Barclay group using Schizochytrium and Thraustochytrium strains.
220. The Detailed Description of the Invention starts at [0016] by repeating [0012].
221. [0017] expands upon the points about the resulting lipids having a low anisidine value, indicating a low level of oxidation. Taking care to limit oxidation was routine in SCO production. [0018] explains that it is the cell walls of the biomass that are degraded by the protease.
222. [0019] refers to additional use of a surfactant, in addition to the enzyme, to liberate the lipids. Dr Kyle’s evidence was that surfactants were routinely used both for this reason (in the context of lysing cell membranes) and for a different reason (namely as an antifoaming agent). Mr Dueppen agreed with both aspects: they were routinely used as antifoaming agents, and also it was CGK that surfactants could be used to help solubilise hydrophilic compounds. It was common ground that the role of the surfactant contemplated by EP155 is not as an antifoaming agent, but in concentrations that assist with cell lysis.
223. [0020] returns to the use of proteases, saying that they provide an economical and simple way under mild conditions to release lipids, which can then be isolated by centrifugation. It is said that in some cases, the lipid will be incorporated into an emulsion, which for some applications might be the final product, but for others the lipid will be recovered from the emulsion. Beyond saying in [0021] that the protease can help break down emulsion-stabilising proteins, the skilled person is not assisted by EP155 with methods to break an emulsion. The patent assumes they have the CGK to do so.
224. EP155 then asserts at [0021]:
In addition, the successful use of a protease for lipid liberation from microalgae is surprising because, microalgae tend to have a low protein content (∼15-22% compared to ∼55% for E. coli), and have very robust cellular structure due to the presence of silica and polysaccharides such as cellulose.
225. It is convenient at this point to mention [0028], which makes a similar point specifically in respect of Schizochytrium:
The lipids are effectively liberated from Schizochytrium sp [i.e. species from the Schizochytrium genus] organisms by treating the cells with a protease enzyme. It is surprising that this particular class of enzymes is effective for this organism due to the relatively small amount of protein normally found in the cell wall of this organism.
226. An issue arose over these expressions of surprise in EP155. In order to understand the evidence on this, it is necessary to refer to some of Dr Kyle’s evidence. Dr Kyle said that the skilled person would not have considered it surprising at all that protease enzymes could be used in this way. He pointed out that it was well known that they have been used to break down yeast, fungal, plant and algal cell walls.
227. As to the suggestion of a low protein content, Dr Kyle said that the skilled person would not have agreed this was generally true of algae or of the numerous different strains of Schizochytrium in particular. While some strains may have a low protein content, the skilled person would not have accepted that Schizochytrium strains contained less protein in their cell walls than similar genera such as Thraustochytrium or Crypthecodinium. To the contrary, if a skilled person was working with a Schizochytrium species they would have known (or would have found out) that these microbes had cell walls comprised of carbohydrate and protein (Kyle 3 ¶¶7-10). It is also wrong as a matter of fact that they had low protein content (Kyle 2 ¶27).
228. Furthermore, even taking [0021]’s figure of ~15-22% protein on its face (which Dr Kyle considered would be read as a reference to the cell wall content, as in [0028] - Kyle 1 ¶182), Dr Kyle said this would not have discouraged the skilled person from using a protease (Kyle 1 ¶184).
229. Dr Wynn disagreed. He said that the protein content of Schizochytrium cell walls was not CGK, nor would it have been a topic of interest to the skilled person (Wynn 2 ¶¶14-18, 24). Nevertheless, he suggested that the Skilled Microbiologist would have known that microalgae in general “would have a low protein content for the reasons stated in EP 155” and “would have considered the microalgal cell wall to be majority carbohydrate” (Wynn 1 ¶¶174, 178). In his reply report, he suggested that the “Skilled Microbiologist would agree with the assumption in [0021]” (Wynn 2 ¶35).
230. The issue of protein content of Schizochytrium arises again in the context of the prior art. However, in summary Mara’s position was that EP155 does no more than apply a CGK technique (namely enzymatic lysis, using a protease - also disclosed in the prior art) to a CGK source of PUFAs (namely Schizochytrium), in an obvious way. EP155 seeks to justify this supposed contribution on the basis that Schizochytrium strains are said to have an unusually low protein content in their cell wall, so lysis by a protease is said to be surprising. However, this is not what the skilled person would have known or expected from their CGK, and it is not in fact true. EP155’s supposed contribution is therefore illusory.
231. [0025] suggests particular strains of Schizochytrium to use, namely ATCC 20888 and ATCC 20889. These were strains first identified by Bill Barclay of OmegaTech (cf the Barclay prior art mentioned above), and at least the first of them was CGK at the priority date, being the strain used to produce OmegaTech’s commercial DHA oil (DHA Gold, subsequently renamed DHASCO-S) (Kyle 1 ¶168; Wynn 1 ¶98). EP155 refers to US patents for information regarding such algae, including the Barclay prior art.
232. DSM were defensive about the % protein figure reported in EP155 for Schizochytrium and were particularly keen to draw attention to [0027] and [0028]. [0027] begins:
‘For different oil-containing materials, different enzymes and reaction conditions can be employed. For these different materials, an important enzyme selection criterion is to select an enzyme that will attack and degrade a portion of the material (such as the proteins, polysaccharides, cell wall, cell outer membrane, peptidoglycan layer, cellulose, chitin, hemicellulose, lignin, lignin-related compounds, etc.) that is otherwise impeding recovery of the oil. Preferably, nonspecific protease enzymes such as trypsin, chymotrypsin, or the like are used to degrade protein components of the oil-containing materials and carbohydrase enzymes such as amylase can be used to degrade carbohydrate components of the oil-containing materials.’
233. DSM submitted that what this is saying is that for enzymatic lysis, it is necessary to consider both the composition and structure of the cells (in particular the cell walls) and to select an enzyme that, in light of the structure and composition, degrade enough of the right part of the cell to release the oil inside.
234. DSM continued by submitting that, in that context, i.e. considering the cell structure and composition, [0028] says that as a matter of fact, a protease enzyme will effectively lyse Schizochytrium cells:
‘The lipids are effectively liberated from Schizochytrium sp organisms by treating the cells with a protease enzyme. It is surprising that this particular class of enzymes is effective for this organism due to the relatively small amount of protein normally found in the cell wall of this organism.’
235. The final part of DSM’s submission was this: “In a sense, it does not matter whether the statement about the amount of protein normally found in the cell wall is right or wrong. What matters is the teaching that the structure and composition of Schizochytrium cells is such that proteases will in fact work.”
236. [0029] goes on to acknowledge that the result of enzymatic lysis may be an emulsion requiring additional treatment, for example, using a polar solvent to aid separation.
237. [0031] returns to the subject of surfactants, again emphasising that the role of protease in combination with surfactant is to recover lipid from a biomass (as opposed to any other purpose), and a long list of possible surfactants is given.
238. There was a dispute over what the Examples showed which went primarily to the insufficiency plea. The issue was whether the examples, and in particular Examples 1 and 3, demonstrate that a protease such as Alcalase is able to cause lysis without the “help” of a surfactant, but the insufficiency plea was dropped so I can deal more straightforwardly with the Examples.
239. I will start by summarising what EP155 actually says.
240. Example 1 reports an experiment in which different combinations of a broad-spectrum commercial protease (Alcalase), a commercial carbohydrase (Viscozyme) and a common surfactant (polysorbate 80) were tested to determine the degree of lysis visible under a microscope after incubation with fermentation broth of an unidentified Schizochytrium species. The results are given in the following table (row numbers added):
241. The results show that use of the surfactant in the absence of an enzyme does not cause any lysis (row 1), and nor does Viscozyme, either alone (row 5) or in combination with surfactant (row 2).
242. In contrast, when the enzyme Alcalase is used in combination with surfactant (row 4), virtually all the cells are said to be lysed. When Viscozyme is added to this combination, the cells are said to be ‘mostly lysed’ (row 3). When Alcalase is used on its own, without surfactant, the cells are reported as being ‘mostly unlysed’ (row 6).
243. The subjective and qualitative terms used for lysis leave some doubt as to what exactly they indicate.
244. While [0039] says that Example 1 demonstrates successful lysis with enzymes and improvement of lysis with the surfactant polysorbate 80, Dr Kyle pointed out that the results indicate that surfactant is essential for substantial lysis (Kyle 1 ¶191). Mr Dueppen disagreed with this characterisation, and points to Example 3 to support lysis by enzyme alone (Dueppen 2 ¶52). It is not necessary to resolve this debate, since it went only to sufficiency.
245. Example 2 uses an approach similar to row 3 from Example 1, i.e. with all three of Alcalase, Viscozyme and a surfactant. However, this time three different surfactants were tested: polysorbate 80, sodium lauryl sulfate (SLS) and dimodan CO-K. Under the incubation conditions used (75 şC for 5 min, then mixing overnight at room temperature), the polysorbate 80 and dimodan CO-K samples are reported to have shown about 100% lysis, while the SLS sample showed about 40-60% lysis. [0043] attributes SLS’s lack of success to it attacking the enzymes - a conclusion with which the experts agree (Kyle 1 ¶192; Dueppen 2 ¶53).
246. Dr Kyle describes Example 3 at Kyle 1 ¶¶193-196. In summary, it compares levels of oxidation of lipid obtained by two methods of extraction from a dried Schizochytrium biomass, one involving enzyme in aqueous solution, the other involving hexane extraction. The enzymatic method uses the protease Alcalase. After incubation with enzyme, the sample is mixed with isopropanol before centrifugation to recover the lipid phase.
247. Claim 1 reads as follows:
1. A method for obtaining a polyunsaturated fatty acid-containing lipid, comprising the steps:
a. providing a biomass which comprises microorganisms of the genus Schizochytrium, said biomass containing a polyunsaturated-containing fatty acid;
b. contacting said biomass with an enzyme; and
c. recovering said lipid,
wherein said step of contacting said biomass with an enzyme comprises treating said biomass with a protease.
248. Claim 5 claimed treating the biomass with a combination of a surfactant and a protease. Although certain points were made to me in opening concerning claim 5, the closings made clear that I was not asked to decide anything regarding claim 5.
249. Although Mara dropped their case that EP155 was insufficient, it was clearly pleaded to keep DSM ‘honest’ when it came to obviousness. In the cross-examination of both Mr Dueppen and Dr Wynn, reference was made to the evidence each gave as to the sufficiency of EP155.
250. In effect, the suggestion was that their evidence on sufficiency of EP155 was inconsistent with what they were saying on obviousness based on Bijl. Although I reject these suggestions, it is worth explaining what they said on sufficiency, which can be compared with what they said in cross-examination on Mara’s obviousness contentions.
251. Each expert included a short section addressing alleged insufficiency of EP155 in their first report.
252. The points which Mr Dueppen addressed concerned surfactants. The first point was whether the Skilled Team would understand that the teaching of EP155 was that a surfactant was required, in addition to a protease, in order to achieve cell lysis. His evidence was clear (and not challenged) that EP155 does not teach that a surfactant is necessary, merely that it would allow milder reaction conditions - i.e. aiding enzymatic lysis. The second point was whether it was plausible that all surfactants would aid enzymatic lysis. His evidence was that it was, subject to doing a routine confirmatory test. He explained that in Example 2, where the degree of lysis was significantly lower, the result is explained as being caused by the sodium lauryl sulfate attacking the enzyme itself. He also explained that routine tests would be run with different amounts of surfactant to arrive at a suitable concentration.
253. Dr Wynn addressed Mara’s allegation that not all proteases would work to break open the cell walls of Schizochytrium. His first point that the final phrase of claim 1 of EP155 ‘treating said biomass with a protease’ would be understood by the Skilled Team as treatment with any suitable protease. His second point was that the Skilled Team ‘would be able to identify the types of proteases that would be suitable using routine testing from the teaching in EP155’. The cross-examination of Dr Wynn emphasised the importance of the words ‘from the teaching in EP155’.
254. I can now turn to consider the question of what the Skilled Team would do, having read and considered Bijl, and the contentions whether EP155 was obvious over Bijl. Again, there are some preliminary topics to discuss.
The dispute as to the approach of the Skilled Team to Bijl
255. Mara accused DSM of taking an essentially abstract approach to the document, without any particular practical goal in mind. Although Mara dropped Barclay as prior art in the course of the trial, they supported this submission by reference to Mr Dueppen’s evidence on Barclay, where - despite the skilled team being interested in producing an oil - he suggested that what would be of interest would not be producing an oil at all, but the suggestion to use the whole cell extruded product by making ‘paste’ with a cheap substance such as corn, for use as animal feed (Dueppen 1 ¶¶89, 136). That, said Mara, was how DSM’s experts’ written evidence was structured, and how the case was put to Dr Kyle, leading to the suggestion that the only obvious thing to do is seek to investigate whether Bijl’s Examples work as reported.
256. Mara submitted that this amounted to a critique of the document per se, divorced from consideration of what the skilled person with a practical aim would do with it. Including Schizochytrium for the production of DHA in the equation came only as an afterthought on DSM’s approach - it was presented as merely an unnecessary complication in the reproduction of Bijl’s Examples.
257. By contrast, Mara suggested that their approach was grounded instead in the practical aim of the skilled team, which (by definition) is interested in producing a PUFA oil from microbial cells.
258. Whether either of these approaches is correct can only be determined once I have considered what the Skilled Team would do, having read and considered Bijl, but is largely irrelevant for that very reason. However I will keep all these points in mind when I return to consider what the Skilled Team would do, having considered Bijl.
The development of Mara’s case of obviousness of EP155 over Bijl
259. In Mara’s Opening Skeleton [108]-[122], their case of obviousness of EP155 over Bijl was set out, largely based on Dr Kyle’s evidence. The basic steps/contentions were as follows:
i) The disclosure in Bijl is very close to the invention that DSM then claimed in EP155 - if Bijl had mentioned Schizochytrium in its list of strains, Bijl would have been advanced by Mara as a novelty reference’
ii) Bijl’s disclosure was plainly of general application and could have been applied to Schizochytrium.
iii) One of the commercial oils at the EP155 priority date, OmegaTech’s DHAGold, was known to be produced from a Schizochytrium species; this is the oil that was renamed S-type DHA or DHASCO-S after Martek’s acquisition of OmegaTech in April 2002.
iv) Therefore, Dr Kyle said it was trivial to apply Bijl’s teaching to Schizochytrium.
v) Of the list of lysis methods in [0015], enzymatic lysis using a protease is selected.
vi) In Dr Kyle’s view, Bijl simply presents the (standard) list of available options, namely enzymatic, physical or mechanical methods of lysis - and within enzymatic lysis, it makes the CGK suggestion of cell wall degrading enzymes including proteases. Since Schizochytrium was known to have a cell wall containing carbohydrate and protein, a protease would have been one obvious option, according to Dr Kyle.
vii) As to choice of enzyme, Dr Wynn’s view is that the cell wall composition of Schizochytrium as containing protein was not a matter of CGK, and is not something the skilled person would have taken the trouble to find out (Wynn 2 ¶¶17-18; Wynn 3 ¶3). Accordingly he considers that a protease would not be an obvious option, despite being specifically called out in Bijl (Wynn 2 ¶¶50-53).
viii) But even if the skilled person did not already know about the cell walls of Schizochytrium, there were two straightforward routes to finding out that the skilled person would have taken as a matter of course (Kyle 3 ¶10).
ix) First, there is a literature search:
a) Dr Kyle says that the skilled person would (if they did not already know that Schizochytrium cell walls contain carbohydrate and protein) look in the literature (Kyle 3 ¶¶11-27). Dr Wynn considers the skilled person would not look, apparently because this literature would be in the taxonomic field and therefore not the sort of publication that the Skilled Microbiologist would normally read (Wynn 2 ¶¶17-18).
b) Dr Kyle says that the skilled person would find a paper by Darley et al. entitled “Cell Wall Composition and Synthesis via Golgi-Directed Scale Formation in the Marine Eucaryote, Schizochytrium aggregatum, with a Note on Thraustochytrium sp.” (Kyle 2 ¶27; Kyle 3 ¶¶12-16; DJK-20 [D2/20]). It analysed the cell wall of a Schizochytrium species and found that it had at least from 30-43% protein and 21-36% carbohydrate (see abstract and Table 1 [D2/20/234]). This did not account for all of the cell wall contents, so the figures may have been higher. Dr Wynn suggests that Darley’s protein figures are misleading and no more than qualitative (Wynn 3 ¶¶3-6). Dr Kyle disagrees about the reliability of the figures, and says that Darley clearly demonstrates that the cell walls of Schizochytrium and Thraustochytrium have a significant protein component (Kyle 3 ¶¶17-20).
c) Dr Wynn says that, if the skilled person did look, the type of publication that would be found is a 2002 paper by Raghukumar (Wynn 3 ¶¶7-8; JPW-7 [C3/7]). It reports that earlier papers (including Darley, which it cites) showed that “The cell walls contain sulphated polysaccharides, predominantly of galactose or fucose, and proteins” [C3/7/84, RHS] (Kyle 3 ¶¶24-26).
d) Dr Wynn also says that the skilled person would most likely find a 1999 paper by Honda (Wynn 2 ¶18; JPW-5 [C3/5]). Dr Wynn points out that, while Honda cites Darley, it mentions only carbohydrate and not protein. Dr Kyle explains that this is because it was the carbohydrate that was of interest to Honda’s taxonomical point. The skilled person reading Honda, and interested in the cell wall composition of thraustochytrids, would follow up the references on this subject, including Darley (Kyle 3 ¶22).
x) Secondly, Mara contended it would be obvious for the skilled person to perform a simple empirical test (Kyle 3 ¶¶28-31). The experiment would have involved adding a range of enzymes to cultures of the particular microorganism to be tested in test tubes, and observing the effect under a microscope. A range of commercial enzymes would have been tested from each of the major classes, for example, proteases, cellulases, hemicellulases, chitinases and pectinases. This would be sufficient to yield information on which enzyme would be most effective to lyse the cells (which would in turn yield information on the cell wall composition). Mr Dueppen’s evidence is that it would be straightforward to test different protease enzymes (Dueppen 2 ¶62).
260. On this basis, Mara’s submission was that the skilled person would therefore either know, or as a matter of routine find out, that a protease was a suitable option to lyse Schizochytrium and claim 1 is obvious.
261. Mara perhaps anticipated that Dr Kyle would face difficulties in cross-examination and so made a determined attempt to establish their case of obviousness of EP155 in their cross-examination of Mr Dueppen and Dr Wynn. This was wise, bearing in mind it became apparent that Dr Kyle’s approach to obviousness of EP155 was heavily tainted by hindsight and, ultimately, as I have indicated above, Mara abandoned any reliance on Dr Kyle’s evidence unless agreed by either Dr Wynn or Mr Dueppen.
262. It was apparent that Mara’s case in closing comprised more or less the same steps as had been set out in their Opening, although the starting point was somewhat truncated, comprising the following steps/assumptions, namely that:
i) DHA is the archetypal PUFA, and there is no dispute that it was (with ARA) one of the most desirable PUFAs at the priority date.
ii) Schizochytrium was one of only two good CGK sources of DHA, and there was unqualified agreement that Schizochytrium would be a good starting point for a DHA oil.
263. Mara therefore suggested that DSM’s experts were cross-examined on the basis of a skilled team with the practical - and entirely obvious goal - of producing DHA from Schizochytrium, in particular Barclay’s strain. In closing, Mara submitted that the evidence extracted from DSM’s experts in cross-examination was ‘resoundingly supportive of obviousness’.
264. As I mentioned above, the evidence of both Mr Dueppen and Dr Wynn provided support for a mindset argument - that in commercial large-scale processing, only mechanical lysis was used or contemplated. This was the basis, as I understand it, for DSM’s argument that enzymatic lysis was not CGK because it was not considered a ‘good basis for further action’ so far as large-scale commercial processing was concerned. This, however, represented a very blinkered and narrow view because it excluded those in the field conducting research in academia and those in industry or elsewhere working at lab-scale on developing or improving processes. For this reason, it is unnecessary to consider the reaction of a Skilled Team only working at commercial scale. They would only consider a process which had been already proved at lab and pilot scale, for scale up to a large-scale commercial process.
265. It is trite that the Skilled Team read each piece of prior art with interest, together with their CGK, and consider what, if anything, they wish to do as a result. Nothing more need to be said about their general approach.
266. The experts were agreed that the Skilled Team would be interested in the teaching in Bijl, albeit for different reasons, leading to very different outcomes.
267. Dr Kyle started his analysis of Bijl with this sentence:
‘Bijl describes the extraction and subsequent isolation of a microbial oil comprising one or more PUFAs directly from microbial cells via an extraction process which uses enzymatic lysis.’
268. As DSM submitted, this opening summary of Bijl is suffused with the hindsight knowledge of where Mara’s case needed to go. The remainder of Dr Kyle’s evidence on Bijl was equally strongly focussed on enzymatic lysis which, in his view, gave the Skilled Team motivation to apply enzymatic lysis to Schizochytrium cells. I give no weight to any of this evidence.
269. Dr Wynn considered that Bijl is of more interest to the Skilled Bioprocessing Engineer than to his Skilled Microbiologist (Wynn 1 ¶199), while Mr Dueppen considered that the Skilled Bioprocessing Engineer would have no reason to enlist the help of the microbiologist in respect of strain choice or lysis method choice (Dueppen 1 ¶140-141).
270. Mara submitted that this was one area where DSM therefore seemed to be trying to exploit its split skilled team, as a way of introducing Nelsonian blindness to Bijl’s explicit suggestion of enzymatic lysis with a protease. This suggestion was made because, in his written evidence, Mr Dueppen differed from Dr Kyle on the CGK nature of enzymatic lysis, so considers that changing the lysis method away from homogenisation would be “an unnecessary distraction” for the Skilled Bioprocessing Engineer (Dueppen 1 ¶140-141). If the Skilled Microbiologist had been involved, Dr Wynn’s evidence was that biological methods of breaking open cells were CGK (Wynn 2 ¶19) and the list of enzymes in Bijl provides a toolkit for researching enzymatic lysis but the Skilled Microbiologist would not know which enzyme would work on which cells (Wynn 2 ¶44).
271. In their written evidence, both of DSM’s experts said that their respective skilled persons would be doubtful about the results of Bijl’s experiments, on the basis that it is said to look unlikely that the conditions used in those experiments would have broken the emulsion that is said to be likely to form. They say therefore that the team’s attention on reading Bijl would be on understanding and replicating its examples, and apparently not on applying its teaching outside the strict confines of its disclosure (Dueppen 1 ¶¶102-103; Dueppen 2 ¶75; Wynn 1 ¶162; Wynn 2 ¶45).
272. Dr Kyle’s written evidence was that the skilled person would not see any problem with the experiments in Bijl (Kyle 2 ¶¶20-25).
273. The difference between the two sets of submissions was that, in the main, Mara’s case was constructed around answers extracted from Mr Dueppen and Dr Wynn in cross-examination, whereas DSM’s case was focussed on attacking any support for it in Dr Kyle’s evidence.
274. The Skilled Team reading Bijl at the priority date of EP155 (May 2002) would, in my view, regard it as a strange document for a number of reasons:
i) First, whilst there was a line of processes in which oils were extracted from intact cells (discussed in the SCO Book), the Skilled Team would regard one of the key statements in Bijl ‘it has now been found that a good quality PUFA oil can be achieved if the cells are in fact lysed’ as significantly out of step with what the Skilled Team would know was happening in the industry in commercial production, where mechanical lysis was used. Indeed, the Skilled Team would recognise that Bijl’s preference for lysis by homogenisation simply reflected what was already the position in commercial production.
ii) Second, of the remaining problems with Bijl which DSM identified, and which I listed out at [212] above, two in particular would, in my view, strike the Skilled Team. First, the absence of any recognition that an emulsion would form and second, the idea that centrifugation alone could break the emulsion formed from mechanical lysis. As to that second point, Mr Dueppen said it would be wonderful if it did, but his view was that the Skilled Team would not believe it would, or, at the very least, be sceptical of the claim.
iii) Third, because of the lysis teaching and the breadth of the claims set out in Bijl, I consider the Skilled Team would conclude that Bijl was an attempt to stake a claim to a wide area of the field, some of which was standard and known (such as mechanical lysis and winterisation), some of which was known at a general level (enzymatic lysis), some of which was desirable but speculative (solventless extraction, separation by centrifugation alone) and some of which ignored the important problem of breaking the inevitable emulsion which would form, particularly if mechanical methods of lysis were used.
275. Consequently, the Skilled Team would be cautious about Bijl and would have to think about whether any part of it provided sufficient motivation to take it forward.
276. As I mentioned above, both DSM and Mara contended that the Skilled Team would be interested in Bijl’s teaching, albeit different parts of it.
277. DSM and their experts suggested that the Skilled Team would be intrigued sufficiently by the suggestion of separation by centrifugation (and without a solvent) to reproduce the two Examples to see if the processes described would actually work. Bearing in mind that in both examples mechanical lysis was used, I conclude that, whilst centrifugation might have resulted in some separation of oil from the emulsion, the amount would be small, such that centrifugation alone would not be seen as worth taking forward, at least for mechanically lysed biomass.
278. A central question on Bijl is whether the Skilled Team would be motivated to apply the suggestion of enzymatic lysis, either at all, or to Schizochytrium microbial cells. Another key question is whether the Skilled Team appreciated that an emulsion from enzymatic lysis would be easier to break than an emulsion generated by mechanical lysis.
279. It is clear that in the course of the trial, Mara’s team sought to develop the proposition that an emulsion formed following enzymatic lysis would be easier to break than a much harder emulsion generated by the ‘violent whacking’ of the mechanical lysis methods. I will be corrected if I am wrong, but I could not discern that any expert volunteered this proposition or said it would have been part of the CGK at the EP155 priority date.
280. However, this proposition was cleverly deployed as the answer if the Skilled Team had any concerns about the separation step - breaking the emulsion - then they would push the Skilled Team towards choosing enzymatic lysis.
281. The closest that the evidence got to this proposition was:
i) Kyle 1, [116] where he said that the advantage of using enzymes to lyse microbial cell walls is that they allow for less harsh reaction conditions, for example lower temperatures.
ii) Kobzeff (but only published in May 2006) suggests that milder pH conditions can be used and also (see [820] below) that the use of a protease can help with breaking an emulsion by breaking down emulsion-stabilising proteins.
iii) I also have in mind that enzymatic lysis would use ‘milder reaction conditions’, as taught in [0019] and [0020] of EP155 itself, but I note also the expression of surprise in [0019] which relates to ‘the use of surfactants with enzymatic treatment can allow for milder reaction conditions’.
iv) This passage in the cross-examination of Mr Dueppen [T2/200/21-201/17]:
21 Q. Can you tell his Lordship, on that issue, surely the use of an
22 enzyme would be far less likely to cause a very well mixed
23 emulsion than whacking it to bits with a homogenizer? I am
24 right, am I not?
25 A. Because we had not done that at that particular time, it would
2 have been unknown.
3 Q. Hardly. A few minutes ago you explained that putting it
4 through a homogenizer was, I think you said something like
5 ripe to create an emulsion. You explained just how likely an
6 emulsion with an homogenizer was?
7 A. Yes, I did.
8 Q. On this score, if you are worried about an emulsion, an enzyme
9 clearly has a potential advantage, does it not?
10 A. Possibly, but unknown.
11 Q. I just do not understand the rationale you put forward to say
12 you are worried about an emulsion, so you stick with the
13 homogenizer; it is completely the wrong way round, is it not?
14 A. I do not agree. We were concentrating on, I know I am
15 repeating myself over and over again, homogenization and
16 knowing whether standard operations were used in that period
17 of time.
282. This passage should probably be read together with the cross-examination on the same issue in relation to Hendrik (in the context of the later 2010 priority date of EP801) at [T3/327/22 - 328/7].
283. As for the first three references, even leaving aside the important point that none of those references evidence CGK at the relevant date, those references rather emphasise the point which also came through in Mr Dueppen’s answers. No-one said that the Skilled Team would spontaneously understand that the emulsion formed from enzymatic lysis would be easier to break than an emulsion from mechanical lysis. If the Skilled Team turned their mind to that specific question, they might well hypothesise that it would and of course, if the Skilled Team started experimenting with enzymatic lysis, they would be likely to discover that the emulsion so formed was easier to break.
284. The problem for Mara is that they could not rely on Dr Kyle’s evidence on obviousness of EP155 and the evidence from Mr Dueppen (and Dr Wynn) was firmly based on their experience in commercial production. I did not receive any (reliable) evidence as to what would have been the reaction to Bijl amongst those accustomed to working at lab scale.
285. In my view, the best way to evaluate this case is to apply the well-known Pozzoli approach. As for the first two stages, I have identified the Skilled Team and their CGK above. The third stage is to identify the differences between Bijl and claim 1 of EP155. The clearest difference is the use of Schizochytrium cells, so I refer to the choice of Schizochytrium as Step 1. A more subtle difference is the bringing to the fore of the use of enzymatic lysis with a suitable protease from (a) the general list in Bijl of CGK methods of lysis and (b) the general list of possible enzymes which could be used in enzymatic lysis, plus the later processing to obtain the desired DHA-rich oil. This would involve the following steps:
i) Step 2: decide to investigate the use of enzymatic lysis.
ii) Step 3A: make a choice as to the enzyme(s) to try.
iii) Step 3B: do a literature search as to whether there was any information as to the cell wall composition of Schizochytrium cells to aid in the selection of a suitable enzyme and/or
iv) Step 3C: perform some lab scale experiments to find out whether certain well-known enzymes would lyse Schizochytrium cells e.g. alkaline proteases, cellulases.
v) Step 4: decide to use an alkaline protease to enzymatically lyse Schizochytrium cells.
vi) Step 5: try the separation techniques taught in Bijl at [0028](f)-(h) and in the Examples to see whether the desired DHA could be obtained with a sufficiently good yield or use other separation techniques.
286. It seemed to me that Mara did not pay much attention to step 5, but I agree that if the earlier steps were obvious, step 5 would not stand in the way of a finding that claim 1 of EP155 was obvious, so I turn to consider the earlier steps.
287. As to Step 1, Mara’s case was developed as follows.
288. First, that Mr Dueppen’s view was that Bijl would be of interest to the skilled person and they would want to take it forward (Dueppen 1 ¶139; Dueppen XX [T2/190/24 - 191/13]).
289. Second, the skilled person is interested in “developing and implementing processes for the productions of [PUFA]-containing oils from microbes” (as Mr Dueppen put it at Dueppen 1 ¶31). The first step in this is strain selection, which Dr Wynn listed as the first role of what he characterised as the ‘upstream’ team (Wynn 1 ¶33a). He described the choice of microbial cell as the “starting point” in manufacturing a PUFA-rich oil in 2002 (Wynn XX [T4/446/19-24]).
290. Third, the desirability of DHA is clear. DHA and ARA were the two PUFAs that were very keenly sought after in microbial systems (Wynn XX [T4/447/22-24]).
291. Barclay’s Schizochytrium strain ATCC 20888 was well-known as a desirable source of DHA, with CGK advantages over the production of DHA from C.cohnii (see [147] above). Both Mr Dueppen and Dr Wynn agreed that Barclay’s ATCC 20888 strain was attractive to use in light of Bijl (Dueppen XX [T2/198/21 - 199/15]; Wynn XX [T4/471/3-12]).
292. Mara’s contention was that the choice of PUFA and microbial source is independent of any particular item of prior art and that it is therefore legitimate to consider a skilled person approaching Bijl with DHA and Schizochytrium in mind. Bijl calls out DHA as a preferred PUFA in [0010] and there is nothing to make the skilled person consider that its teaching is inapplicable to Schizochytrium.
293. As for Step 2, Mara submitted that the method of lysis was in Mr Dueppen’s realm. Dr Wynn also said that Bijl is of more interest to the Skilled Bioprocessing Engineer than to his Skilled Microbiologist (Wynn 1 ¶199).
294. Mara submitted that in this passage of cross-examination, Mr Dueppen agreed that using enzymatic lysis was one of the obvious things to do (Dueppen XX [T2/198/4-13]):
Q. Right. You cannot suggest that using an enzyme to effect cell
lysis would not have been one of the things that would have
been technically obvious to the skilled reader reading this
document, surely.
A. It is a possibility they would have looked at that and said,
"Yes, we could use lysis", but I do not know which lysis,
which enzymes or combination of enzymes to use to lyse the
cells.
Q. When you just said "Yes, we could use lysis" you mean ----
A. I meant enzymes.
295. This was evidence that the Skilled Team could use enzymatic lysis, but the question is whether they would do so.
296. Mara sought to bolster the choice of enzymatic lysis because it would avoid the expense - at least for a start-up company that did not already have one - of investing in a homogeniser, a big one of which could cost $500,000 (Dueppen XX [T2/243/18-19]).
297. Mara also sought to bolster the choice of enzymatic lysis on the basis that it would produce a gentler and easier to break emulsion, a point I discussed at [283] above.
298. As to Step 3A, the Skilled Team would note that Bijl expressly suggests a protease (e.g. an alkaline protease) at [0015]. Mr Dueppen agreed that, when it came to picking enzymes (whether to use on their own or in combination), proteases would be high on the list because proteins were one of the three main components known to make up the cell barrier (Dueppen XX [T2/201/18-24]; see also [150] above). Dr Wynn’s evidence was also that the skilled person would know in advance that Schizochytrium cell walls would contain protein in significant quantities (see above). So a protease such as Alcalase would be identified as an enzyme to try.
299. As to Step 3B, this would involve, for DSM’s Skilled Team, the Skilled Bioprocessing Engineer asking the Skilled Microbiologist to see if more specific information was available.
300. If the answer came back that protein was present at 30-40% of the cell wall, Mr Dueppen merely said that other things might have been of interest as well, but did not disagree that a protease would be attractive to pursue (Dueppen XX [T2/201/25 - 202/19]).
301. If the skilled person did look for information on Schizochytrium cell walls, they would find Darley et al. [D2/20]. Dr Wynn agreed that PubMed was commonly used in 2002 and that a PubMed search for the cell wall composition of Schizochytrium would be routine and would identify Darley (Wynn XX [T4/426/2-3, 498/5 - 500/25]). In fact, looking at just about anything on this topic, they would find Darley since it is cited by practically all the papers in the case on this topic (Wynn XX [T4/501/2-5]).
302. Darley analysed the cell wall of a Schizochytrium species and found that it had at least from 30-43% protein and 21-36% carbohydrate (see abstract and Table 1 [D2/20/234]). Dr Wynn made certain criticisms of the protein figures in Darley’s abstract (Wynn 3 ¶4):
i) First, he drew attention to Darley stating that the levels of carbohydrate and protein as a percentage of dry weight varied widely between different batches (see start of Results section at [D2/2/233]). He suggested that it followed that an accurate determination of these compounds could not be obtained, and the Skilled Microbiologist would therefore understand this paper to be a qualitative rather than quantitative analysis. This makes no sense on its face. What Darley states means no more and no less than that the levels of carbohydrate and protein varied between different batches; as Darley explains in the following sentences, this variability was not found when individual components were examined. It therefore does not reflect experimental error, but real difference between the samples, which Darley postulated may reflect different ages of the cultures.
ii) Dr Wynn then pointed out that Darley’s discussion on page 101 [D2/20/242] noted that “[o]nly 50-60% of the dry weight of the cell walls has been accounted for in this study”, and the authors theorised that a significant portion of the unaccounted dry weight may be sulphate groups on sugars. However, this would merely increase the percentage of weight attributable to carbohydrates on account of the sulphate moieties on them. It does not affect the proportion that was found to be protein.
iii) In the witness box, Dr Wynn had a new point, on arithmetic (Wynn XX [T4/502/22-503/2]). He said that that if 50-60% of weight was unaccounted for, this did not tally well with the data. But Dr Wynn here had mis-read Darley (or his own quotation of it). Darley does not say that 50-60% was unaccounted for. It says that 50-60% was accounted for. This is consistent with the data. The minimum total weight accounted for was 51% (i.e. the sum of the minimum of 21% carbohydrate and 30% protein). Since figures for individual samples are not given, it is not possible to marry up carbohydrate and protein figures to determine what the maximum accounted for was - but if the maximum carbohydrate were paired with minimum protein, that would be 30+30=60%. In any case, I agree that this is a point of meticulous arithmetical analysis to which lawyers are prone.
303. The criticisms of Darley are therefore unwarranted. They clearly did not occur to the numerous authors who cited Darley as a source of information on Schizochytrium cell walls, including Bahnweg and Jäckle, who wrote that “Darley et al. (1973) found that cell walls of Schizochytrium aggregatum and Thraustochytrium sp. were characterised by high protein and low polysaccharide contents, the latter being composed predominantly of galactose and xylose” [D2/27/320].
304. In any event Dr Wynn’s quibbles did not affect the response that he said the Skilled Microbiologist would pass back to the Skilled Bioprocessing Engineer about the results of the literature search. This would have been as follows (Wynn XX [T4/504/11 - 505/5]):
[Q.] …. Are you suggesting to his Lordship that the
skilled person, having been asked by the notional Mr. Dueppen
to do this task, having found Darley, they would then dismiss
it and tell their colleague that they could not find anything?
A. No, absolutely not.
Q. Right. So they would pass on what Darley says?
A. They would.
Q. Which is protein 30-43% and carbohydrate 21-36%; yes?
A. There I think we have a slight disagreement. I think they
would say the protein is likely to be somewhere above 30%, the
carbohydrate content I think is going to be significantly
higher than reported in Darley.
Q. They would definitely pass on what Darley says. Whether they
add to it is another matter. But they would definitely pass
on what Darley says; yes?
A. They would pass on what Darley says. And what Darley says for
me is that there is a protein element to the cell wall and
there is this galactose related, probably sulphated galactose
carbohydrate portion as well.
305. As to Step 3C, Mr Dueppen agreed that it was routine in 2002 to test different reagents, in different quantities, to assess their effect on lysis by looking under the microscope to see if lysis was achieved (Dueppen 1 ¶146; Dueppen XX [T2/101/14 - 103/3]). Such routine tests were used as part of R&D into looking at alternatives to hexane extraction (Dueppen XX [T2/138/28 - 140/2]).
306. The upshot is that the Skilled Team might posit the use of a protease at Step 3A and do some simple tests to see if it worked at Step 3C - i.e. whilst Step 3B was an obvious step, it might be bypassed. By either of these routes, the Skilled Team would reach Step 4.
307. I take Mara’s point that a decision to use enzymatic lysis necessarily entails a decision as to which enzyme to use, and the former decision might not be taken until a chosen enzyme has been shown to work - at least at lab scale. Mr Speck KC was insistent that the steps involved were not Technograph steps, for this reason.
308. Mara’s written closing on EP155 said nothing about the separation of the lipid from the lysed biomass and, as far as I can detect, nothing was said about it in oral closing. It was simply assumed that separation would be possible - as it would be. However, the final key question is whether Mara’s case utilised the separation technique(s) taught in Bijl (i.e. ‘centrifugation, optionally with the addition of one or more salts’) or not. There was no positive indication that Mara’s case did so.
Conclusions
309. It should not be a surprise that the above series of steps can be formulated to lead from the prior art Bijl to claim 1 of EP155, but the key question is whether those are a series of Technograph steps or whether the combination of all those steps was obvious to the Skilled Team in 2002.
310. I have come to the conclusion that EP155 was not obvious over Bijl. Although it is clear that Mr Dueppen and Dr Wynn took somewhat of a negative view of Bijl in their written evidence, those views were ameliorated in the answers they gave in cross-examination. I also consider that Dr Wynn was overly resistant to suggestions that the Skilled Team would be able to decide on a suitable enzyme. If the Skilled Team had decided to investigate enzymatic lysis, they would have found a suitable enzyme or combination of them (e.g. a protease and a carbohydrase). However, overall the evidence extracted from Mr Dueppen and Dr Wynn in cross-examination did not, in my view, establish a case of obviousness for four main reasons.
311. First, when one stands back from the detail, the steps I discussed above show that in fact, Mara’s case uses Bijl as nothing more than a hook to pick up the suggestion of enzymatic lysis. Beyond that, Mara’s case appeared to me largely to discard the rest of the teaching of Bijl, so this case is not significantly different from a case of obviousness over the CGK concept of enzymatic lysis.
312. The related, second, point is that what was new in Bijl (for the Skilled Team) was not the mention of the CGK methods of lysis but the suggestion of a solventless separation step using centrifugation, with the optional addition of one or more salts. This separation step was demonstrated in two examples, each of which featured mechanical lysis. There was no example drawing attention to enzymatic lysis.
313. Third, enzymatic lysis was CGK and the notion had been around for some years. What was unexplained in Mara’s case was why this particular mention of enzymatic lysis in Bijl would trigger the Skilled Team to investigate it, whereas other mentions (e.g. in conferences recorded in the SCO Book) had not. Bijl was published in February 2002, only a few months before the EP155 Priority Date of 3 May 2002. However, in view of the way Mara’s case was developed the question: if it was obvious, why was it not done before? has greater significance, to which no answer was supplied.
314. Fourth, despite Mara’s best efforts, there was no evidence to support their suggestion that the Skilled Team, on reading Bijl, would understand the emulsion ‘problem’ was caused by the violence of mechanical lysis, that enzymatic lysis would produce a gentler emulsion and this thought process would point the Skilled Team towards taking up enzymatic lysis.
315. Overall, it is difficult to avoid the conclusion that there was an undue focus on enzymatic lysis, caused by hindsight.
316. The issue was reasonably finely balanced such that, with better expert evidence (i.e. which gave a reason why the Skilled Team would focus on enzymatic lysis without hindsight), I might well have been able to find that EP155 was obvious, but, on the basis of the evidence I received, EP155 was valid.
EP740
317. Claim 1 of EP740 as granted was very wide indeed, claiming any microbial oil with >50% DHA in TAG, however it was produced:
‘1. A microbial oil comprising a triglyceride fraction of at least 70% by weight, wherein the docosahexaenoic acid content of the triglyceride fraction is at least 50% by weight, and wherein the oil comprises 5% by weight or less of heptadecanoic acid.’
318. DSM applied to amend the claims unconditionally and did not attempt to defend the claims as granted. It relied on what it called claim set B, of which claims 1B and 2B (claiming microbial oils with a mix of product and process features) are said to be independently valid and infringed.
319. The proposed amended claim 1B reads as follows:
‘1B. A microbial oil comprising a triglyceride fraction of at least 70% by weight, wherein the docosahexaenoic acid content of the triglyceride fraction is from at least 50% 55% to 65% by weight, and wherein the oil comprises 5% by weight or less of heptadecanoic acid, wherein the microbial oil is a crude oil extracted from the biomass of a microorganism without further processing, and wherein the microorganism is a thraustochytrid microorganism.
320. Proposed amended claim 2B narrows the DHA range somewhat:
‘2B. The microbial oil of claim 1B, wherein said docosahexaenoic acid content of the triglyceride fraction is from at least 60% to 65% by weight.’
321. All references in what follows are to these amended claims.
322. Claim 1B of EP740 is a product claim, but it contains process steps so this case raises an issue about product by process claims. Two particular process steps are sought to be added by amendment, namely that:
i) the oil is “extracted from … a thraustochytrid microorganism”; and
ii) it is “a crude oil extracted … without further processing”.
Both these relate to the history of the product - what has (or has not) been done to obtain it.
323. All the issues on EP740 (save for insufficiency and added matter) concern these process features of claim 1B.
324. DSM summarised their contentions on EP740 as follows.
325. By reference to amended claim 1B, DSM said that the invention of EP740 is a new, highly desirable crude microbial oil containing high amounts of the essential polyunsaturated fatty acid DHA, and that such an oil is particularly useful in the manufacture of dietary supplements.
326. The high DHA content of the EP740 microbial oil is described in the claims by reference to the DHA content of the triglyceride fraction (the “TAG”), by weight. It is convenient to call this the % DHA in TAG.
327. Having the DHA in the oil in the form of the TAG is important and useful because:
i) Triglycerides are relatively oxidatively stable.
ii) Triglycerides are not removed during refinement processes.
iii) PUFAs in breast milk are in TAG form, so it is beneficial to mimic this characteristic in a supplement that is present in infant formula.
328. Claim 1B claims an oil having 55-65% DHA in TAG. DSM submitted this is a significantly higher percentage of DHA in TAG than any previously known crude microbial oil
329. DSM say that Mara have never disputed that the % DHA in TAG feature is a highly beneficial feature of the oil of the invention. Mara have a specific product, a crude oil which it calls Mara DHA Plus, to draw attention to the fact that it has a high DHA content. Mara admit that certain batches of DHA Plus have a % DHA in TAG within the range specified in claim 1B (and in some cases, also claim 2B) and it is not disputed that those batches infringe those respective claims.
330. The claims also specify that the oil has a triglyceride fraction by weight (“% TAG”) of at least 70%. It is common ground that this was a feature of existing crude microbial oils, including crude Thraustochytrid oils. It is present in the claim because the DHA content is specified in terms of % DHA in TAG, and therefore this additional requirement limits the claims to oils with high absolute quantities of DHA.
331. DSM point out that Mara do not actually allege invalidity on the basis that the skilled person could make an oil having all the features of the claims at the priority date, however much they might want to. DSM say that Mara’s prior art case has always been based on saying that certain integers of the claim are irrelevant and trying to have them written out of the claim.
332. For their part, Mara summarised their position as follows.
333. EP740 describes a screening program for thraustochytrids, reminiscent of that carried by Professor Barclay. Mara say that DSM found one good strain and that if DSM had sought claims corresponding to their actual technical contribution, many of the problems with EP740 would not arise - the underlying point being that Mara do not use the strain that EP740 discloses.
334. Underpinning Mara’s case is the submission that DSM claimed too greedily and the problems cannot now be avoided because DSM has tried to claim the whole class of oils per se, no matter how made and regardless of EP740’s contribution.
335. Mara oppose the application to amend on the grounds of added matter and lack of clarity. The Comptroller considers the amendments are not allowable. So, one might think the first issue is whether the proposed amendments are allowable.
336. Leaving that issue aside, Mara also say EP740 is plainly invalid for insufficiency, on the basis that EP740 only discloses a single strain that produces an oil that falls within the claims (with supposedly a higher percentage of DHA than in oils from previous strains). Yet EP740 claims all oils with the claimed characteristics, despite giving no help with finding other strains that produce such oils.
337. Finally, Mara say that EP740 is invalid for obviousness over prior art called Fabritius, once the new product-by-process features are ignored (as Mara say they must be).
338. All these points are disputed by DSM.
339. As will appear, the parties approached the issues on EP740 in different ways and they chose to deal with what turned out to be the same issue in different parts of the case.
340. I propose to deal with the different parts of the case in the following order:
i) Set out the agreed additional CGK for EP740 and deal with two CGK disputes.
ii) Explain the disclosure of the single piece of prior art relied on by Mara - Fabritius.
iii) Explain the disclosure of EP740.
iv) Address the correct characterisation of claim 1B. Nearly all of the disputes concerned this point. It may be noted that the issues under this head also arose in the guise of the majority of Mara’s objections to DSM’s application to make the amendments shown in claims 1B and 2B.
v) Once claim 1B has been correctly characterised, the issues which are dependent on it are readily resolved. These are obviousness over Fabritius and Amendment.
vi) Finally, I deal with Mara’s insufficiency and added matter attacks.
341. Mara say that the correct characterisation of claim 1B leads to the conclusions (a) the amendments to the claim must be refused and, relatedly (b) even if one assumes the amendments have been made, the process features of the claim are impermissible and must be ignored.
342. In response to Mara’s argument that the process features of claim 1B must be ignored for the purposes of obviousness, DSM pursued the following three lines of argument. The significance of each ebbed and flowed as the evidence developed and some were combined, but I introduce these lines of argument here:
i) First, DSM attempted to establish that the process features of claim 1B related to a characteristic of the product (the oil) itself. Mara detected three separate attempts by DSM to establish this but say that all failed.
ii) Second, DSM argued that Mara were addressing the wrong question and said the correct question was whether claim 1B had a technical effect over the prior art.
iii) Third, DSM chose to ignore any problem with the process elements by treating the lack of clarity objections in a very literal fashion - contending that the additions to the claim were clear.
343. It is convenient to start with the additional CGK for EP740 over that already stated for EP155.
344. This section sets out the agreed areas of CGK which applied at the EP 740 Filing Date, namely 19 March 2009, in addition to the matters set out as CGK for EP155 above.
345. By 2009 there was a greater interest from different companies in exploiting microbial oils, as an alternative to fish oil, and the relative proportion of PUFA oils commercially produced from single cell oil sources increased significantly.
346. Between 1973 and 2003 an estimated 2,100 metric tons of microbial oils were sold worldwide. Between 2004 and 2008 around 13,500 tons of microbial oils were sold, with 2008 alone accounting for 3,500 tons. Figure 14 below is an extract from the second edition of Single Cell Oils, Chapter 20, which shows the relative proportion of different microbial oils from 1973 to 2008. As the charts show, the vast majority of the microbial oils sold were DHASCO (C.cohnii oil) and ARASCO oils (Mortierella alpina oil), which were the chosen microbial oils for infant formula during this time period.
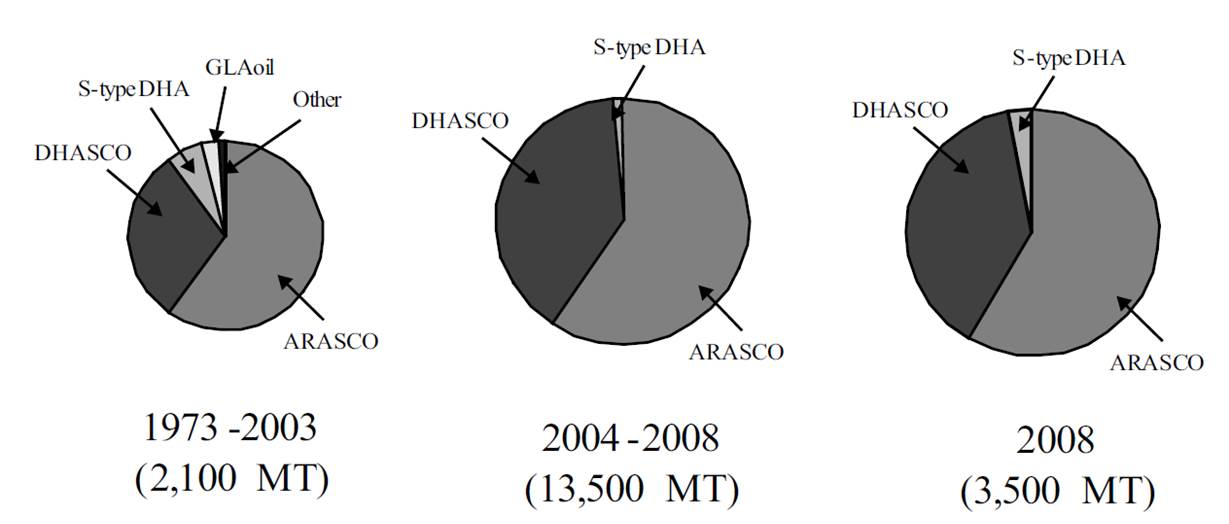
Figure 14: Relative production of microbial oils
347. The relative sales volume contributions of DHASCO and ARASCO (i.e. more ARASCO than DHASCO) reflects the 2:1 ratio recommendation of DHA:ARA in the Martek infant formula supplement.
348. The issue was identified by Mara as follows: Whether what mattered to the skilled person in respect of an oil was the composition of the oil, or the fact that it had come from a particular organism.
349. As both sides appeared to agree, this is not a point about CGK at all. Instead, it is a question which arises in the context of Mara’s objections to the amendment to reach claim 1B, requiring the oil to be derived from a Thraustochytrid microorganism. Both sides agreed the question is best addressed in that context, which I do below.
350. The meaning of winterisation, and in particular whether the conditions in Fabritius could be considered to fall within the common understanding of winterisation. [Dueppen 1 ¶62; Wynn 1 ¶224; Kyle 1 ¶¶127, 221, 224, 228; Dueppen 2 ¶¶39-41; Wynn 2 ¶¶76, 78; Kyle 2 ¶28]
351. Mr Dueppen agreed in cross-examination that winterisation involves cooling so that compounds with a higher melting point solidify and can be removed from the liquid - for example, removing saturated fatty acids. It works by differential crystallisation which occurs on cooling.
352. The issue turned on whether the label ‘winterisation’ only applied when the temperature is around zero degrees, or whether it also applied at lower temperatures (and/or in the presence of solvent). Fabritius itself at [0001] described its technique as ‘winterisation’. Mr Dueppen preferred the term ‘extreme winterisation’ on account of the presence of solvent and the extreme temperatures, but winterisation nonetheless (Dueppen XX [T2/212/7-13]). Therefore, in the end there was no dispute that Fabritius’s technique is properly to be recognised as a variety of the CGK technique, with the CGK label.
353. Mara submitted there was no dispute as to what Fabritius disclosed. Despite that, the parties were keen to emphasise or draw attention to different matters.
354. The teaching is summarised in [0001]:
‘The present invention relates to a method for producing natural PUFA-enriched PUFA-triglyceride mixtures ... having a minimum PUFA content of >55% by weight of TFA (Total Fatty Acids), the great majority of those consisting of triglycerides. These are obtained by winterization in one or more organic solvents from natural PUFA oils having a PUFA content >39% by weight of TFA.’
355. In [0009]-[0015], Fabritius describes some existing challenges with increasing the concentration of PUFA in TAGs in an oil, including the CGK enrichment techniques applied to marine oils.
356. [0023] states that it is an object of Fabritius’ invention to provide a method for “producing natural highly concentrated PUFA-triglyceride mixtures from PUFA oils of lower concentration” containing inter alia DHA.
357. The process is summarised in [0027]-[0032] and expanded on in [0033]-[0068].
i) The process starts with a PUFA oil (which can be derived from a variety of microorganisms including Schizochytrium or Thraustochytrium [0034]) having ≥85% TAG and >39% PUFAs (of total fatty acids). The PUFA oil is dissolved in an organic solvent or mixture of solvents.
ii) The “winterization” step involves the mixture standing at a temperature between -35°C and ‑100°C (see [0029] and [0045]). The experts agreed that this differs from the commonly used winterisation process in the CGK but disagreed about whether this is enrichment or refinement. However, nothing turns on this dispute.
iii) The solid fraction is then separated from the liquid supernatant.
iv) Finally, the solvent is removed from the supernatant, leaving the highly concentrated PUFA triglycerides.
358. The Fabritius method produces an enriched oil which is said to always have “a total PUFA content of >55% by weight of total fatty acids” - see [0037].
359. As mentioned above (in the CGK section for EP740), there was a dispute between the experts as to whether the Fabritius process should properly be called a winterization process. However, what is clear is that it provides oil with a PUFA content, specifically DHA, of greater than 55% by weight of total fatty acids.
360. The points which emerged in cross-examination were as follows.
361. Although Mr Dueppen had not been asked to address Fabritius in his written evidence (except on the point about the meaning of ‘winterisation’), he agreed that the document fell on his side of the divide between DSM’s two experts (Dueppen XX [T2/216/11-16]). He was therefore cross-examined on it, and Mara submitted that his views supported Mara’s obviousness case (see generally Dueppen XX [T2/211 - 240]).
362. Mr Dueppen agreed that the DHA percentages in Fabritius’s examples are a good guide to the percentage of DHA in the triglyceride fraction (Dueppen XX [T2/237/25 - 238/7]). Mara submitted it was agreed that this percentage by weight of total fatty acids could be taken as indicating the percentage by weight in TAG - also pointing out that DSM was content to take the % in total fatty acids as indicative of % in TAG because they needed to do the same in reliance on the data in EP740 as regards the mutants in Example 7. Accordingly, Fabritius discloses oil with a DHA in TAG over 55%, and in many cases over 60%, by weight.
363. Mara submitted that there seemed to be some confusion in the cross-examination of Dr Kyle as to the temperature at which the higher melting point TAGs will crystallize out differentially, due to the fact that Fabritius has solvent included - something that Mr Dueppen was alive to when explaining why he referred to it as ‘extreme winterization’ (see [T2/212/7-10]). A substance that is in a solvent which is cooled such that it crystalises out of solution will do so at a temperature that is dependent upon its concentration and solubility in that solvent. It does not crystalise out at the melting temperature of the pure substance. The effect of the solvent was raised by Dr Kyle too in that cross-examination [T6/797/25 - 798/2]. The precise temperature at which it occurs (or more properly starts to occur) does not seem to matter but, as Mara submitted, it obviously cannot be at 66şC for the tri-palmitate TAG, as seemed to be being suggested. If it were, there would be no need to cool at all.
364. So far as the Examples are concerned, DSM were keen to emphasise that each one was a very small-scale laboratory experiment using Fabritius’ method, which produce even smaller amounts of concentrated oil (<1 gram). In each case a solvent comprising a mixture of hexane and either ethanol or acetone is used, and the mixture is frozen for varying amounts of time at -85°C. The DHA content is assessed as a percentage by weight of total fatty acids (“TFA”):
i) Example 1 starts with 1g of commercially available DHASCO oil, which has a DHA content of 45.1% by weight of TFA. Mara submitted that it was well known that DHASCO had a triglyceride fraction of >95% (a figure that Mr Dueppen thought reasonable [T2/233/19 - 234/2]). The DHASCO oil is dissolved in a solvent. The freezing is at -85°C for 8 hours. The material is then centrifuged to separate the frozen portion from the supernatant, and the solvent is then removed. The result is that the DHA content of the oil in the supernatant increased to 57.5% of total fatty acid, albeit only 0.377g of oil is produced.
ii) The other 3 examples all use an oil “produced as described in Yokochi et al”. Dr Wynn and Dr Kyle disagreed about whether it would be clear from that paper exactly how this starting material oil was produced. Nevertheless, it was common ground that it must be an oil derived from a Schizochytrium strain. It is said to have a DHA content of 47% (or 46.4%) by weight of TFA.
iii) In Example 2, using a procedure similar to that in Example 1, 1g of that oil produces 0.537g of oil with a DHA content of 64% by weight of TFA, the procedure being similar to that in Example 1.
iv) Example 3 used a longer freezing time and a different solvent mixture, which resulted in an oil with 70.1% DHA.
v) Different solvents were investigated in Example 4, giving rise to DHA in the range 60-69%.
365. In the arguments relating to EP740, it will be seen there is frequent reference to an oil which has passed through Fabritius Example 2.
366. The parties disagreed as to EP740’s disclosure. DSM contended that EP740 is directed to Thraustochytrid oils. Specifically, that it focusses on a new Thraustochytrid microbial oil, which is a crude oil product, characterised by a high triglyceride fraction and high amounts of DHA in that triglyceride fraction.
367. By contrast, Mara contended that EP740’s disclosure is directed to the use of a new species in the thraustochytrid order - PTA-9695 - to produce a microbial oil with commercially desirable features. EP740 explains that PTA-9695 is most closely related to Barclay’s ATCC 20888 strain - see Table 2 and [0077].
368. Those commercially desirable features are a high level of DHA in the TAG fraction of 55 to 65% (leaving aside the dispute over clarity which I deal with below). The benefit of this - as the skilled person would understand it - was explained by Dr Wynn at ¶250(2) of his first report: it enables the same amount of DHA to be delivered in a smaller volume of oil. He gave the example of producing a nutritional supplement capsule to be swallowed.
369. Mara contended there is nothing particularly special about 55 to 65% (or indeed 60 to 65%). Dr Wynn confirmed that a higher proportion of DHA is generally an improvement on a lower level - [T4/532/2-4]. So even higher would be even better.
370. The specification begins with a description of the background art, explaining some basics of fatty acids in [0002], PUFAs and long chain PUFAs i.e. LC-PUFAs in [0003], the importance of omega-3 PUFAs in [0004] and the disadvantages of existing sources of such PUFAs (flaxseed and fish oils) in [0005]. [0006] then introduces Thraustochytrids. With the citations omitted and emphasis added:
[0006] Thraustochytrids are microorganisms of the order Thraustochytriales. Thraustochytrids include members of the genus Schizochytrium and have been recognized as an alternative source of omega-3 fatty acids, including DHA. Oils produced from these marine heterotrophic microorganisms often have simpler polyunsaturated fatty acid profiles than corresponding fish or microalgal oils. Strains of thraustrochytrid species have been reported to produce omega-3 fatty acids as a high percentage of the total fatty acids produced by the organisms. However, isolated thraustochytrids vary in the identity and amounts of LC-PUFAs produced, such that some previously described strains can have undesirable levels of omega-6 fatty acids and/or can demonstrate low productivity in culture. As such, a continuing need exists for the
isolation of thraustochytrids demonstrating high productivity and desirable LC-PUFA profiles.
371. The Detailed Description starts with these paragraphs, where the words ‘Thraustochytrid Microorganisms’ which appear at the end of [0011] appear to have been misplaced and are a heading for [0012]-[0018] i.e.:
[0011] The present invention is directed to a microbial oil as defined in claim 1.
Thraustochytrid Microorganisms
[0012] The disclosure is directed to isolated thraustochytrids, including mutants, recombinants, and variants thereof.
[0013] In some embodiments, the disclosure is directed to a thraustochytrid of the species deposited under ATCC
Accession No. PTA-9695. The isolated thraustochytrid is also known herein as Schizochytrium sp. ATCC PTA-9695.
372. As explained below, EP740 discusses some mutant strains of ATCC (American Tissue and Culture Collection) PTA-9695 which were also deposited as PTA-9696, PTA-9697 and PTA-9698, but [0017] makes it clear that these mutant strains were all derivatives of PTA-9695.
373. [0018] makes clear what EP740 means by a fatty acid profile:
[0018] In some embodiments, an isolated thraustochytrid of the disclosure, including mutants, variants, or recombinants thereof, comprises a fatty acid profile in one or more fractions isolated from the thraustochytrid. The one or more fractions isolated from the thraustochytrid includes the total fatty acid fraction, the sterol esters fraction, the triglyceride fraction, the free fatty acid fraction, the sterol fraction, the diglyceride fraction, the polar fraction (including the phospholipid fraction), and combinations thereof.
374. The next relevant heading is Microbial Oils, containing [0028]-[0032], followed by Compositions (i.e. comprising a microbial oil of the invention) [0033]-[0049] and ‘Methods of Using the Compositions’ [0050]-[0062] and Kits Comprising the Compositions’ to [0070]. Examples 1-8 then comprise most of the remainder of the specification, followed only by ‘Sequence information’. Example 1 seeks to establish that ATCC PTA-9695 is a new Schizochytrium species. Example 2 discusses cell growth of ATCC PTA-9695 under varying culture conditions. Examples 3-6 are all concerned with microbial oils extracted from the particular isolated thraustochytrid ATCC PTA-9695. Example 7 concerns mutants of PTA-9695. Example 8 appears to be comparative because it utilises four thraustochytrid samples from the ATCC unrelated to PTA-9695. In each of Examples 3-8 there are extensive tables of Fatty Acid Profiles (Tables 5-29), the relevant details of which I discuss further below.
375. Having set out the basic structure of the specification, I can now turn to the particular parts to which the parties drew attention.
376. Mara submitted that the disclosure of EP740 is very clear that the advantages it is putting forward can be achieved by an oil however it comes to have the beneficial features, citing [0029]. This is an important paragraph in several respects, and it is necessary to quote it in full, in which I have underlined certain phrases for emphasis:
[0029] The invention is further directed to a microbial oil comprising a fatty acid profile of the invention. A microbial oil of the invention can be any oil derived from a microorganism, including, for example: a crude oil extracted from the biomass of the microorganism without further processing; a refined oil that is obtained by treating a crude microbial oil with further processing steps such as refining, bleaching, and/or deodorizing; a diluted microbial oil obtained by diluting a crude or refined microbial oil; or an enriched oil that is obtained, for example, by treating a crude or refined microbial oil with further methods of purification to increase the concentration of a fatty acid (such as DHA) in the oil.
377. Mara’s point was that this central teaching in EP740 is clear: the advantage can be achieved however the oil is obtained. It can be a crude oil extracted without further processing, a refined oil, a diluted oil obtained by diluting a crude or refined oil, or an enriched oil. However, the additional point to note is EP740 expressly teaches that the oil in question (of the invention) has a fatty acid profile, albeit in the context of any likely claim, one might expect to see a fatty acid profile expressed in ranges of individual fatty acids.
378. Dr Wynn accepted that all these oils mentioned in [0029] - including the diluted oil - were all equivalent in the context of this advantage of delivering an amount of DHA in a smaller volume of oil [T4/535/25 - 536/6].
379. Mara then turned to certain of the Examples. Example 2 of EP740 grows the cells in different conditions - specifically varying salinity. It is the lowest salinity conditions (those in [0087]) that are taken forward to Example 3, which takes cells grown in those lower salinity conditions, extracts the oil in two different ways and subjects the oil to analysis. The methods of extraction are hexane extraction and FRIOLEX (see [0088]), producing Samples A1 and A2 respectively.
380. Results for each sample are in fact provided in two separate tables - Table 5 and 6 for Sample A1 (hexane extraction) and Table 7 and 8 for Sample A2 (the FRIOLEX extraction oil). Dr Wynn did not know if there was any significance in the slight differences between the numbers obtained for the different samples - [T5/542/17 - 544/6].
381. As Mara submitted, the results in the respective tables need to be handled with some care. They present measurements of the levels of a fairly long list of fatty acids in the biomass, in the crude oil and in various fractions, for example TAG and free fatty acids. But not everything was included - for example phospholipids, as Dr Wynn agreed. And they do so in two different ways - hence there are two tables for each sample. The first table in each pair - Table 5 and Table 7 respectively - presents the results for each fatty acid as mg/g - i.e. the milligrams per gram of the fraction (crude oil, TAG, free fatty acid etc). The relevant extract of Table 5 and 7 for DHA (C22:6 N3) is as follows:
Table 5
Table 7
382. Table 5 shows the content of DHA for Sample A1 was 569.7 mg/g in the crude oil and 556.5 mg/g in TAG. Table 7 shows for Sample A2 the DHA content was 566.7 mg/g in crude oil and 575.0 mg/g in TAG. These values are very close to each other and are, in percentage terms, 57% DHA in the crude oil (which ties in with the value reported at [0087] for Example 2 where the same low salinity conditions were used) and 56-57% DHA in TAG.
383. An alternative presentation of results is in Tables 6 and 8. What has been done here is to take the mg/g measurement from Tables 5 and 7 respectively and, instead of presenting it as a percentage of the total weight of e.g. the crude oil or TAG, it is presented as a percentage of only all those fatty acids that were measured. The Sum of All FAME’s at the foot of the rows in Tables 5 and 7 have been used (Wynn XX at [T5/548/24 - 550/23]. These Sum of All FAME’s numbers are expressed in mg/g and are all less than 1000 - reflecting the fact that not everything in the crude, TAG etc has been accounted for.
384. Extracts from Tables 6 and 8 for DHA are as follows:
Table 6
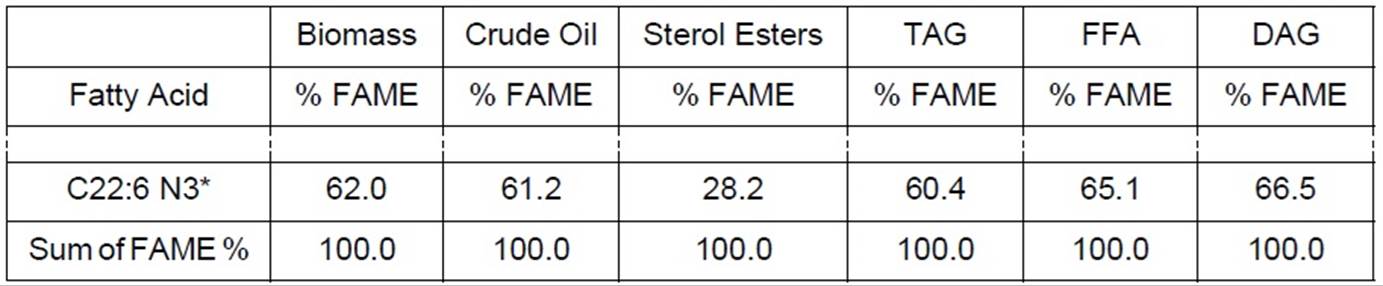
Table 8
385. As can be seen, the percentage values for DHA in crude oil and TAG are higher when presented this way - as Dr Wynn accepted [T5/550/24 - 552/2]. The percentage of DHA in TAG is now given as 58.1% (for Sample A1) and 60.4% (for Sample A2) and the DHA in crude oil has also risen to 61.0% and 61.2% for Samples A1 and A2 respectively. The difference between the two samples arises predominantly because the Sum of All FAME’s total for the TAG fraction of Sample A2 is significantly lower than for A1. Mara were keen to point out that neither of the Sample A1 nor A2 figures in Table 6 and 8 is actually the percentage of DHA in TAG - that is actually seen in Table 5 and 7 by looking at the mg/g measurement - 56% and 57% respectively.
386. In their opening skeleton DSM appeared to only rely upon Example 3 so far as EP740 discloses anything in accordance with the invention is concerned - see EP740 skeleton ¶¶16-22 on EP740 disclosure, where only ¶21 refers to anything potentially relevant - which is Example 3. DSM drew attention to both the overall % TAG and the % DHA in TAG. Thus, Sample A1 has a % TAG of 82.7% and % DHA in TAG of 58.1%. Sample A2 had % TAG of 83.4% and % DHA in TAG of 60.4%.
387. DSM submitted that this therefore provides an example of a crude oil with a high triglyceride fraction and high amounts of DHA in that triglyceride fraction being achieved from a Thraustochytrid. This is considerably higher than any crude oil from a Thraustochytrid before the priority date, as Dr Kyle accepted. DSM drew the comparison with oil from ATCC 20888 (which was used by OmegaTech to produce its DHA-rich oil) which was reported to have 41.7% DHA in TAG.
388. Nonetheless Mara also addressed Example 7 of EP740, as they detected that DSM now wished to rely on it. It concerns PTA 9695 cells that have been subjected to UV irradiation to cause slight variations in DNA. This process is known as mutagenesis. What Example 7 involves is carrying out this process and then recovering individual cells, growing them in colonies, using each colony to grow up a sample in a fermenter and then analysing the fatty acid profile (Wynn XX [T5/553/6-18]).
389. Tables 15 to 21 set out the results for many mutants. At least 74 were created - although not all have been reported in the EP740.
390. The data in Tables 15 to 21 do not actually report DHA in TAG specifically. Rather the data is a percentage of the entire fatty acids (Wynn XX [T5/554/17 - 555/2]). Of those reported the vast majority show a % DHA of total fatty acid at around 55-58 percent. The table prepared by the Claimants’ lawyers at [CXX-DJK/4] conveniently arranged them in descending order so that the spread can be seen. PTA-9695 itself is shown highlighted (DHA of 56.88%), around which the vast majority sit, in the range 55-58%. There are a very small number a little bit higher and a little bit lower than that range at 53/54% and at 59/60%.
391. As can be seen in [CXX-DJK/4] right at the bottom there is one as low as 41.61%. And the top 3 are 61.42%, 61.83% and 62.23%.
392. Dr Wynn’s first report dealt with these data with a very light touch, merely noting the entirety of them in a very short paragraph dealing with Example 7 (Wynn 1 ¶262). Dr Wynn said nothing about the sufficiency issues in his first report. His second report included just 2 paragraphs directed to sufficiency (¶¶73 and 74), neither of which touched on the levels of DHA enabled by EP740. In XX, Dr Wynn agreed that the results for mutants 73 and 74, both around 62%, were - if we take the DHA percentage of total fatty acid as indicative of DHA content in TAG - as good as it gets [T5/562/13-23]. If the mutants data cannot be used, the only results in EP740 are those in Example 3 - those seen in Tables 5 and 7 - 56% and 57% DHA in TAG.
393. I have already set out the proposed amendments above. As appears from the underlining, the integers sought to be added are
i) To limit the DHA in TAG to be in the range 55 to 65 % by weight (assuming in DSM’s favour on meaning and clarity) or in the case of claim 2B to the range 60-65%;
ii) To limit the oil to one that is a crude oil extracted from biomass without further processing; and
iii) To limit the microorganism to one that is one in the thraustochytrid order.
394. DSM addressed the construction of each of these additions.
395. As to the first, DSM submitted the added words define a range of 55% to 65% (in claim 1B) and 60% to 65% (in claim 2B). DSM acknowledged that the words “at least” were unnecessary but submitted they do not cause any real difficulty in understanding what the patentee means. DSM also submitted that nothing turned on the level of precision used in the claim; but if it mattered, they said the figures are to be treated as precise to the nearest whole number. Mara’s clarity objection raised a separate point which I consider below.
396. As to the second, DSM relied on the general process for the production of a crude microbial oil product as set out schematically in Kyle 1 Figure 12 (taken from the SCO Book). Subject to one qualification, it was common ground that a “crude oil ... without further processing” means the product that is output from the “Oil Extraction” step, i.e. following lysis and lipid separation, prior to further downstream processing. The further downstream processing (i.e. the further processing steps that are excluded) were part of the agreed CGK and are set out above.
397. DSM characterised one of Mara’s arguments as a point on the (literal) clarity of this expression, although I understood Mara’s argument to be different. So far as what may be termed ‘literal’ clarity is concerned, DSM submitted that Dr Kyle had absolutely no difficulty with the term “crude oil” or what it means. Similarly, Dr Wynn was clear that the characteristics of claim 1B in a crude oil “adds value to this crude oil as a product” [Wynn XX T4/528/9-24].
398. As to the third, the meaning is clear, but, again, Mara’s clarity objection raises a different issue.
399. The purpose of the limitation to DHA in TAG to the ranges of 55-65% and 60-65% was, so Mara contended, to deal with the issue of sufficiency that EP740 had not enabled high levels of DHA in TAG. The point is to remove the higher levels from the claim. Mara said the issue remains as to whether DSM has enabled the top end of either of these new ranges.
400. The purpose of the ‘crude oil/without further processing’ limitation was to seek to distinguish oils with the claimed commercially desirable characteristics (i.e. TAG level and DHA level in TAG) which is obtained by one or more processing steps.
401. Mara submitted that there were two separate, but linked, issues that arose on this aspect of the proposed amendment. Mara said that the first issue that arises is whether the limitation is actually adding a characteristic of the product at all. If it does not, then Mara submitted it cannot be relied upon for novelty or obviousness as a distinction over prior art, and on that basis Mara submitted the claims are invalid over Fabritius. The second issue is that if the wording does import a characteristic of the product itself (rather than its history), then does it fall in the narrow exception where product by process claims are allowable?
402. Finally, DSM said that the purpose of the limitation to the microorganism being a thraustochytrid is to seek to narrow the breadth of claim as to the different cells it covers. Mara submitted it is not much of a limitation - the claim is being narrowed from covering every microorganism possible to every microorganism within the entire thraustochytrid order. The consequence is that DSM have to establish enablement across this breadth. Mara said that this feature also introduces issues as to whether this truly refers to a characteristic of the oil and, if so what it might be.
403. Reverting to the point made by Mara in [401] above, I agree that those issues are linked because they both concern the nature of claim 1B and how it should be characterised. As Birss J. explained in Hospira v Genentech [2014] EWHC 3857 (Pat), product by process claims are tricky and this case seems to involve two closely related aspects. The first concerns the approach to validity of product by process claims. The second concerns the allowability of the amendments which seek to introduce the process elements.
404. As already mentioned, claim 1B of EP740 is a product claim, but the two particular process steps sought to be added by amendment are:
i) the oil is “extracted from … a thraustochytrid microorganism”; and
ii) it is “a crude oil extracted … without further processing”.
Both these relate to the history of the product - what has (or has not) been done to obtain it.
405. Mara relied on two principles of law, which I accept are established by the case law I cite below:
i) First, an identical product does not count as new if made by a new process, even if that process is written into the claim.
ii) Second, the same applies to a product that is identical to an obvious product.
406. The impact on validity of product by process features was considered by Lord Hoffmann in Kirin-Amgen v TKT [2004] UKHL 46, [2005] RPC 9 at [86]-[101]. This decision was reviewed and explained by Birss J (as he then was) in Hospira v Genentech [2014] EWHC 3857 (Pat) at [125]-[147]. Mara drew attention to the following key passages:
‘138. In Kirin-Amgen the House of Lords had to consider the novelty of an overt product by process claim. This is dealt with in the speech of Lord Hoffmann at paragraphs 86 to 101. A number of points arise. Lord Hoffmann dealt with the history of product by process claims and noted that the advantage they had before the 1977 Act was removed by s60(1)(c) (paragraphs 88-89). He noted that the idea that a process could confer novelty on a known product was not particularly logical since the history by which it was made is not an attribute which it carries around and makes it new (paragraph 88). He dealt with the EPO’s practice starting from the 1980s, referring to the IFF/claim Categories T150/82 decision and the EPO’s practice (paragraphs 90-91). He was puzzled by an earlier decision of the EPO relating to the patent in suit which appeared to be based on inconsistent findings of fact as to whether the process of making recombinant erythropoietin (rEPO) did or did not necessarily give rise to differences with known urinary erythropoietin (uEPO) (paragraphs 92-95) and noted that the trial judge (Neuberger J as he then was) had found as a fact that there was no necessary distinction between rEPO and uEPO (paragraph 96).
139. In Kirin-Amgen the Court of Appeal had held that the product by process claim (claim 26) was novel because of the novel process feature. The Court of Appeal had refused to follow the EPO’s practice about permitting such claims only in certain circumstances because that was a rule of practice of no concern to national courts. Lord Hoffmann (with whom the other lords agreed) did not agree with the Court of Appeal’s reasoning (paragraphs 98-101). He held that a difference in the method of manufacturing did not make a product new and that was so as a matter of law. On that basis the claim could only be novel if the process definition gave the product a new characteristic of some kind. On the finding of fact in Kirin-Amgen, therefore claim 26 lacked novelty since the process did not necessarily do so. The decision of the Court of Appeal was wrong. The UK should follow the approach of the EPO.’
407. Birss J therefore at [140] identified the ratio of the decision in Kirin-Amgen as being that an identical product made by a new process does not count as new and noted that in that respect the UK now follows the EPO.
408. He went on to point out that the House of Lords did take the process feature into account when determining infringement, from which he concluded that treating a product by process claim as claiming a product per se (without regard to its processing history) is not a matter of construction, but a feature of the law of novelty. Birss J also drew attention to the impact on obviousness, which is not a point that arose in Kirin-Amgen.
‘145. The result is that a product not made by the claimed process has been found not to infringe because it was not made by the claimed process while another product not made by the process has been found to render the claim lacking novelty despite the fact it was not made by the process. This is a little paradoxical but it shows the difficulties one can get into with product by process claims. A further puzzle is the following. What if, in Kirin-Amgen, the prior art uEPO had not been disclosed so as to be relevant for novelty but was something which was obvious? Presumably it would make the claim obvious for the same reason?’
409. Birss J. concluded his analysis of the legal principles as follows:
‘147. I derive the following principles from this consideration of the EPO and UK authorities:
i) A new process which produces a product identical to an old product cannot confer novelty on that product. To be novel a product obtained or obtainable by a process has to have some novel attribute conferred on it by the process as compared to the known product.
ii) This rule is a rule of the law of novelty. It is not a principle of claim construction. Although in effect the rule treats “obtained by” language as “obtainable by” language, nevertheless as a matter of claim construction a claim to a product “obtained by” a process means what it says. That will be the relevant scope of the claim as far as infringement and sufficiency are concerned.
iii) Although normally a patent is drafted by the inventor “in words of his own choosing”, the EPO will not permit overt product by process language unless there is no other alternative available. By no other alternative, they mean no other way of defining a particular characteristic of the product in question.’
410. Finally, it is helpful to note some observations made by Birss J in [157] when applying the principles to the facts of the case before him (emphasis added):
‘[157]…. Since the EPO’s practice runs counter to the idea that a patentee is entitled to use words of his own choosing in describing his invention, it must be based on some principle. The principle underlying the EPO’s practice is shown by the Johnson Matthey case. It is a principle of clarity (Art 84 EPC, s14 of the 1977 Act) and amounts to a trade off between clarity and fairness, tolerating an increased lack of clarity in that limited class of cases. If a patentee can identify a characteristic or parameter disclosed in the patent for which no other definition is available in the specification other than an “obtainable by” process definition, then a product by process claim may be allowed as a way of claiming that attribute. It is impossible to apply that approach properly without knowing what characteristic the process feature is to be used to define. That would be best stated in the claim expressly but it may be clear from the specification.’
411. Mara submitted that, as Birss J. recognised in [145], it follows from the logic of Kirin-Amgen that the approach to novelty should also apply to obviousness. They submitted this must follow since if an old product anticipates a product by process claim because the process by which it is obtained is irrelevant, then if the same product is obvious, it will render the claim obvious.
412. Mara submitted that this is because the law, in both the EPO and the UK, is that a “patentee who wishes to complain of dealings in a product made by his patented process must rely on his process claim and article 64(2)” (or s.60(1)(c) in the UK) - see Lord Hoffmann in Kirin-Amgen at [90]. Mara’s case is that is exactly what DSM has not done with its product claim.
413. I did not understand DSM to dispute the underlying principles. Instead, they disputed the application of them to the facts in this case.
The developments in this aspect of the case
414. It became increasingly evident that the parties were taking different approaches to the issues raised by the process elements sought to be introduced into claim 1. There were two principal consequences. First, in the way the evidence developed through the trial and second, the approaches taken in closing submissions.
415. It will help to explain the developments if I outline the principal difference between the parties as reflected in their closing arguments.
416. DSM simply addressed the objections of lack of clarity and added matter. DSM did not address the prior issue concerning the process elements of these product claims, notwithstanding the effort which DSM expended on evidence which was aimed at this issue (see further below). As far as I could tell, at least in their written closing, DSM simply ignored the issue by asserting (a) there was no difficulty with the term ‘crude oil’ and (b) the characteristics of claim 1B in a crude oil added value to it as a product - i.e. an argument on technical effect.
417. Mara stressed that it is necessary to have the correct nature of the argument well in mind. As Mara submitted, it is not a question of whether the amendment provides a technical advantage that can be relied upon for the purposes of inventive step as against a particular item of prior art. If the point was whether there was a technical effect, it would not be being suggested, for example by Birss J, as applicable by extension of logic from the lack of novelty case. Mara therefore characterised the issue as being different and more fundamental: whether the words provide any definite distinction over an old (or obvious) product.
418. These issues developed in the following way.
419. In their Opening Skeleton, DSM addressed Mara’s Statement of Objections to the proposed amendments, including the contention that the requirement that the microbial oil is “a crude oil ... without further processing” has no technical effect. DSM dismissed this point as not a ground for invalidity and treated it as an allegation that the addition represented an arbitrary feature and so could not be relied upon to defend an obviousness attack, but also submitting the objection was without merit. That was all that was said by DSM on technical effect, at that stage, and DSM’s oral opening made the same points as in its Skeleton. On that amendment, DSM took the position that the words were completely clear.
420. Mara set out their case on EP740 quite fully in their Opening Skeleton argument, including their contentions as to why claim 1B was neither clear nor concise, addressing each of the three proposed additions. On the second - ‘a crude oil extracted…without further processing’ and third (Thraustochytrid) Mara referred to and relied on this passage from Dr Wynn’s second report (Wynn 2 ¶¶67-68) and then made the submissions in [233] and [234]:
‘‘…different microbial oils are derived from different microorganisms i.e. Thraustochytrid oils are derived from Thraustochytrid microorganisms and Crypthecodinium oils are obtained from Crypthecodinium microorganisms. The resulting oils produced from these different sources are fundamentally different products with different physiochemical properties that alters oil stability and organoleptic characteristics (i.e. taste/flavour). The Skilled Microbiologist can identify the differences between them by examining the characteristics of the oil.
68. For example, the Skilled Microbiologist would know the type of the microorganism determines the fatty acid profile of the oil and could analyse this characteristic. The simplest way to differentiate between Thraustochytrid and Crypthecodinium oils is to analyse for the presence of DPA, as this fatty acid is not present in Crypthecodinium oils.’
[233] Dr Wynn is therefore saying that an oil derived from a thraustochytrid can be identified by examining its characteristics - for instance, the fatty acid profile differs. In that case, it follows that the product can be characterised by reference to its structure or composition. The claim therefore does not fall within the narrow exception to the prohibition on product by process features, and the claim is bad for lack of clarity or conciseness.
[234] The situation is similar for the “crude oil… without further processing” feature, which again is a process feature. It appears that DSM may seek to suggest that “crude oil” is a feature of the product per se, and therefore legitimate to claim as such, but this cannot be right since it would require all “crude oil” to be distinguishable (without looking at its history) from all oil that has been processed even to a small extent.’
421. So it seemed clear that DSM were arguing that the words refer to a distinguishing characteristic of the product (the oil). Mara therefore submitted that must mean that all oils that are crude oils can be distinguished from all oils that are not crude oils and posed this question: what then is it that this limitation is said to bring to the oil?
422. In closing, Mara said they had detected at least three attempts by DSM to come up with a basis for arguing that the limitation relates to a characteristic of the oil itself rather than a process limitation and addressed each one in turn.
423. The first attempt was to suggest that processing steps render the oil chemically distinct. I was told that DSM attempted to insert this into the agreed CGK, but Mara objected. In their Opening Skeleton, DSM suggested it was the substance of the CGK anyway - see EP740 skeleton footnote 12, which also referred to Mr Dueppen’s first report ¶63, which suggested that this feature (or possibly “crude oil” alone, since DSM accepts there is redundancy in the wording) has the effect that the oil that is claimed is physically and chemically distinct from an oil that has undergone further processing steps (DSM skeleton ¶53).
424. Mr Dueppen was the first witness and Mr Speck KC, after asking some questions about RBD processes, including winterization, then turned to explore whether one could distinguish between a crude oil and a refined oil, the product of one or more of those processing steps [T2/114-115]:
17 Q. So if I gave you a sample of the starting oil before any of
18 these processing steps had been undertaken, and a sample that
19 had been subject to one or more of these processing steps, you
20 would, given enough time and resources to test them, be able
21 to tell me which was which; yes?
22 A. Yes, I would.
23 Q. That is what you are referring to in paragraph 63 of your
24 first report, where you say: "A refined oil is chemically
25 distinct from a crude oil"; yes?
2 A. Yes.
3 Q. The crude oil in that sentence in line 2 is a reference to the
4 specific oil from which the specific refined oil you are
5 referring to was derived?
6 A. Yes, the starting material would have been derived from the
7 same starting material as the finished refined oil.
8 Q. It is a completely different matter to being able to tell for
9 any oil at all that I give you whether it has or has not been
10 subject to any of these processes to any degree; agreed?
425. Mr Dueppen asked for the question to be repeated. After some clarification I can pick up his answer at p116:
3 But I could typically tell
4 between a crude oil and a refined oil, which is which, based
5 on the analytical composition of the material.
6 Q. Indeed, if I give you both?
7 A. If you give me both and they are from the same source, with
8 the analytical techniques that we typically use in the
9 industry, we would be able to tell the difference.
10 Q. If I give you both. It is absolutely crucial that I give you
11 both, is it not?
12 A. It is, as long as, again, the timeline is not that this
13 refined oil has been sitting around for a long period of time.
426. Mr Speck KC then reverted to an example:
19 If I gave you an oil with a level of phospholipids in it, you
20 would not know whether it had been subject to that to get it
21 down to that level or not, would you?
22 A. If I did not know what the starting material was, that would
23 be correct, yes. I would have to have the beginning and the
24 final to look at the difference.
25 Q. Unless you actually know the history, you just cannot tell
2 from an individual oil, can you?
3 A. You cannot tell what?
4 Q. Whether it has undergone any of these processes.
5 A. If you do not know the starting material, that would be
6 correct. You would assume that, again, if it has gone through
7 a refining step, it has less phospholipids or soaps than the
8 final material.
427. Thus, in cross-examination Mr Dueppen readily accepted that one could only tell a crude oil apart from a processed oil if one had both of them to compare, in other words if one knew the history of the oil. Simply given an oil on its own, it would be impossible to say whether it was a “crude oil” or a processed oil. The whole passage of XX is at [T2/113/25 - 117/8].
428. On the basis of these answers, Mara submitted that DSM cannot suggest that “crude oil” (or “crude oil … without further processing”) is a product feature -being a “crude oil” does not impart a feature to the product - one simply cannot tell from the product whether it is a crude oil or not.
429. Those answers were given on day two. At [26]-[27] above, I described how those answers led to the service of Wynn 4.
430. As appears below, DSM’s answer to this passage of cross-examination was to assert that it was not relevant to the case on Fabritius, invoking their ‘technical effect/benefit’ argument.
431. Wynn 4 represented DSM’s second attempt to justify their position, prepared in the knowledge of what had happened in Mr Dueppen’s cross-examination.
432. The essence of the approach in Wynn 4 was to confine consideration only to thraustochytrid oils that are either (a) a crude oil or (b) a crude oil that has been through Fabritius Example 2. Whilst accepting for the purpose of the argument the contention that it is only oils from thraustochytrids that count, Mara made the point in closing that this is still a massive class of potential oils.
433. Nonetheless when confined to this situation, Dr Wynn suggested if given any oil sample which was one of (a) or (b), he could tell whether it was (a) or (b). He relied upon the level of two particular TAG molecules with wholly saturated fatty acids in each of the 3 fatty acid positions.
434. Mara contended this addressed the wrong question, but submitted that, in any event, the contention did not survive cross examination.
435. Mara submitted that Wynn 4 addressed the wrong question because it is confining consideration only to whether all and any oil that had been through Example 2 of Fabritius could be distinguished from all and any crude oils. Mara pointed out that the correct question is whether the purported limitation is a feature of any oil at all.
436. DSM sought to get round this by saying the point is a point on technical effect and inventive step - thus arguing that particular prior art can be brought in to the question. But, as I outlined above, Mara’s position (supported by the dicta of Birss J.) was that it is not a point on technical effect at all and more fundamental than that - it is whether the limitation is a limitation to the product (the oil at all) i.e. whether it is a limitation regardless of whether it has a technical effect.
437. In closing, Mara submitted that the problems with Dr Wynn’s evidence that emerged in cross examination were as follows:
i) He assumed the thraustochytrid oil extracted but not processed (i.e. the crude oil) would always have a level of TAG molecules with the saturated fatty acids of those seen in the EP740 example - i.e. of around 1% or a little higher - see Wynn XX [T5/582/18 - 583/14].
ii) Dr Wynn also suggested that another approach was simply to put an oil in the fridge and if it went cloudy he would conclude it was a crude oil. Otherwise, it would be identified as an oil that had been through the Fabritius Example 2 process. In this instance he said he was not limited in his view to the EP740 oil example but he was considering ‘pretty much any crude oil’ [T5/584/8 - 586/5, but especially T5/585/12]. Mara submitted that is not good enough - the argument must be he can tell in the case of every possible crude oil from every possible thraustochytrid that has gone through Fabritius Example 2.
iii) Dr Wynn had not thought about what the cut off was - i.e. the level of the saturated TAG molecules above which the oil would be a crude oil and below which it would be identified as having been through Fabritius Example 2 - he gave an ‘off the top of the head’ answer of certainly well below 1% and another equally unreasoned answer of 0.5% - see [T5/588/2-17].
iv) What was clear is that the fridge approach was not testing for the same level - and therefore not the same thing as the negligible criterion, whatever that actually was - see [T5/598/15-24].
v) Dr Wynn had assumed that no thraustochytrid oil could ever have levels of the saturated TAG molecules significantly higher than the 1% level of the EP740 strain [T5/589/11-20].
438. When pressed further on this he eventually revealed that his evidence was focusing on thraustochytrids that produced 55-65% DHA in their crude oil - see [T5/592/3-24]. That explained why he was seeking to contend that crude oils would not have levels of the saturated TAGs significantly higher than the 1% he had assumed. I agree with Mara that that last point was fatal to Dr Wynn’s evidence on this point and DSM’s Attempt 2.
439. However, by confining consideration to only thraustochytrids which produce 55-65% DHA Dr Wynn was excluding all those oils from thraustochytrids which are below 55 to 65% DHA in the crude oil but which would or could be brought to be within that range by the application of Fabritius. Mara pointed out that this was the very point of the Fabritius validity attack.
440. Mara submitted that this showed that Attempt 2 failed even when limited to considering crude oils with oils that have been processed in accordance with Fabritius Example 2.
441. Indeed, it emerged in Dr Wynn’s cross examination that this was his answer to the example given by Dr Kyle in ¶12 of his fourth report. Dr Wynn had no disagreement with the suggestion that the oil, after going through Fabritius Example 2, would have levels of the saturated TAG species above negligible levels - his answer was it was not an instance where the crude oil has DHA levels between 55 and 65% - see [T5/596/10-24].
442. I reserve my decision on Attempt 2 until later.
443. Mara submitted that, having seen Dr Wynn’s fourth report fall apart in XX, counsel for DSM attempted a third approach in the course of XX with Dr Kyle. Mara made the point that it had no basis in any of DSM’s evidence - even the very late-filed Wynn 4.
444. Like Attempt 2, this argument was also constrained to considering crude oils as against those that has been through Fabritius - so again, Mara submitted it addressed the wrong question, for the reasons set out above.
445. The approach of Attempt 3 was to focus on the relative proportion of the TAG molecules containing DHA. A TAG molecule obviously has three fatty acids. So, a TAG comprising DHA could have 1, 2 or 3 DHA fatty acids. These are called monomer, dimer and trimer as regards the DHA content. Of course, a DHA monomer TAG could also be a dimer of another fatty acid - whether saturated or unsaturated. Or a monomer of two other different fatty acids, saturated or unsaturated.
446. The cross examination started at [T6/774/9] - by focussing on the Fabritius process removing some DHA.
447. But as Dr Kyle explained, the DHA being removed could be in any form, not necessarily in TAG - he gave examples at [T6/774/20-23]: free fatty acids, wax esters and sterols, all present in significant amounts. He also reiterated that you do not know what the make-up of the oil is [T6/776/3-5].
448. After cross examination seeking to establish the basis for Attempt 2, Mr Abrahams KC returned to this topic at [T6/777/8]. The proposition put at [T6/777/18-20] was that if the oil had ‘plenty of trimers of DHA, plenty of dimers of DHA but no monomers of DHA we would know it had been through the Fabritius process’. But Dr Kyle explained it depends upon what was in the starting material and there is an enormous variation in the starting oils [T6/777/21-24].
449. Mr Abrahams KC tried again - this time by postulating specific information about the starting material and end material - and suggesting the mass that is removed will be, or include, the monomers of DHA; Dr Kyle thought the saturated fatty acids would be removed - [T6/778/12 - 779/11].
450. Mr Abrahams KC tried again from [T6/779/12-20] where Dr Kyle said the high DHA content oil with monomers, dimer and trimers of DHA would not change after the Fabritius process.
451. Undeterred it was suggested that the turbid residue in the Fabritius process will contain monomers of DHA [T6/780/3], which Dr Kyle did not accept - he said they could be saturated fatty acids.
452. Dr Kyle did also say if there was a particular DHA monomer - that with two C16 saturated acids - that would probably come out, but he went on to say a lot of other things would come out before that - see [T6/780/4-12].
453. Dr Kyle was very clear that if there is DHA in whatever form - monomer, dimer and trimer - they are going to be the last things to freeze out: [T6/780/13-16].
454. The final attempt was at [T6/781/3-17] where the point put was again - Dr Kyle accepted the ‘first thing’ (meaning first TAG containing DHA) that will fall out would be a monomer with the other two positions saturated, as opposed to TAG with all 3 positions DHA.
455. Mara submitted that this did not go nearly far enough to be able to provide a basis to argue that every oil that goes through Fabritius Example 2 can be distinguished from every conceivable thraustochytrid oil that is crude and not processed. It did not even attempt to give an indication of the separate levels of TAG molecules that are DHA monomers, dimers and trimers or the relationship between each of those three levels that would be used to decide whether any given oil was a crude oil or one that had been through example 2 of Fabritius, let alone identify them with any precision. Mara pointed out this was not surprising as all these contentions came from counsel in cross-examination without any evidence upon which to base the point.
456. Mara also pointed out that attempt 3 assumed the monomers of DHA are - in all conceivable oils - in TAG that have saturated acids at other positions. They posed the rhetorical questions: what about those with significant levels of monomer where the other fatty acids are not those particular saturated acids, or not saturated acids at all? what about the removal of DHA being removal of those other non-TAG components Dr Kyle referred to at the start of the XX? or oils with high levels of TAG in DHA trimer and dimer to start with?
457. In short, Mara contended that Attempt 3 - not thought of by either of DSM’s experts or supported in their evidence - also failed to provide a basis to be able to distinguish all crude oils from all oils that have gone through Fabritius Example 2 - even if it were the correct question.
458. As foreshadowed above, DSM had two answers to Mara’s contentions regarding Attempts 2 and 3. DSM said they overlapped and mixed them together:
i) One was, again, to rely on the technical benefits of the crude oil of claim 1B over an oil that could be produced by Fabritius Example 2.
ii) The other was an argument that the Fabritius Example 2 process does not produce an oil which is identical to the crude oil of the invention.
459. Mara’s argument of obviousness of claim 1B over Fabritius can be succinctly stated.
460. First, Mara submitted that Fabritius would be of interest to the skilled person for the following reasons:
i) As Fabritius states at [0018] and Mr Dueppen agreed, there was a great requirement for natural triglycerides which are highly enriched with PUFAs (Dueppen XX [T2/224/22 - 225/3]). Dr Wynn described a high-triglyceride oil with high DHA in the triglycerides as commercially desirable (Wynn 2 ¶84).
ii) Dr Kyle explained that there was an interest in using highly enriched DHA oil in pharmaceutical applications (Kyle 2 ¶31).
iii) Mr Dueppen’s view was aligned with that of Dr Kyle; namely the higher concentrations of DHA achieved by Fabritius would be beneficial in pharmaceutical applications (such as for cystic fibrosis that Fabritius specifically identifies), so as to increase the amount of DHA that could be administered in a capsule, and would be feasible at that scale (Dueppen XX [T2/213/19 - 216/2, 224/4-17]).
iv) Mr Dueppen agreed that the skilled person would want to use Fabritius’s method for a pharmaceutical application (Dueppen XX [T2/239/7-11]).
461. On this basis, Mara submitted that it would have been obvious to follow Fabritius’ teaching. Mr Dueppen agreed that for a pharmaceutical application, the skilled person would be keen to make an oil in accordance with Examples 2, 3 or 4 (Dueppen XX [T2/239/19-24])
462. The oil would have the features of the claim, as the limitation as to crude oil/further processing steps is not a limiting feature of the oil as I have found above.
463. Mara’s final point was that even if the ‘crude oil’ distinction is a feature of the product as opposed to the process, it provides no technical effect - it is an arbitrary feature that cannot be taken into account for inventive step.
464. DSM contended there was only one issue on Fabritius which they chose to frame as follows:
i) Mara’s case is that it would be obvious to take a Thraustochytrid oil and subject it to the Fabritius process, thereby producing an enriched Thraustochytrid oil with >70% TAG, 55-65% DHA in TAG, and <5% HDA.
ii) But such an oil would not be a “crude oil … without further processing”.
iii) This was the reason, DSM submitted, that Mara pleaded, at SOO ¶10A, that this requirement of claim 1B lacks a technical effect and so cannot be relied on.
465. On this basis, DSM submitted that Mara’s argument is wrong in fact and law.
466. In Takeda v Roche [2019] EWHC 1911 (Pat), [2019] RPC 18, [203], Birss J referred to the principle that the patent monopoly should be justified by the actual technical contribution to the art, and went on to explain at [204]:
One way in which this principle has been applied in the context of inventive step is to deny validity to a selection from the prior art “which is purely arbitrary and cannot be justified by some useful technical property”. Such a selection “is likely to be held to be obvious because it does not make a real technical advance”. These passages are taken from Floyd LJ in Generics UK Ltd t/a Mylan v Yeda [2013] EWCA Civ 925, citing Jacob LJ in Dr Reddy’s Laboratories (UK) Ltd v Eli Lilly and Co Ltd [2010] RPC 9.
467. Birss J clarified how this works in practice in Optis v Apple [2020] EWHC 2746 (Pat), [206]-[209] and his conclusion was approved by the Court of Appeal [2021] EWCA Civ 1619, [58] (Arnold LJ):
The principle is not that a claim which contains an arbitrary feature [i.e. a feature which lacks technical effect] is invalid. Merely having an arbitrary feature in a claim is not a ground of invalidity. The point of Agrevo obviousness is that if a claim is found to contain an arbitrary limitation in it, then that limitation cannot assist the patentee in defending an obviousness case. The claim still does have to be obvious over something in the state of the art - perhaps common general knowledge or some cited prior art.
468. For these reasons, DSM submitted that the issue does not arise as a free-standing question divorced from any prior art. It arises only in the context of an allegation of obviousness over a cited item of prior art, or the CGK, in which case the question is whether the distinction over that art has a technical effect or, in the alternative, is arbitrary.
469. DSM proposed an illustration: consider a claim with two features, A and B, and two cited items of prior art. As against the first prior art, feature A may be a difference but one that has no technical effect and is therefore arbitrary; but the patent is not obvious because it has feature B. As against the second prior art, feature B may be a difference but one that has no technical effect and is therefore arbitrary; but the patent is not obvious because it has feature A.
470. DSM submitted that what the illustration shows is that asking whether a feature has a technical effect or is arbitrary, in a vacuum, is an irrelevant and meaningless question (at least in the context of obviousness). The question is whether the claimed invention has a distinguishing feature, which has a technical effect, over the matter forming part of the state of the art.
471. Further, DSM submitted that every patent would be invalid if a defendant could posit a thing which was not part of the state of the art and say that the claimed invention had no technical benefit over it.
472. This is why, so DSM submitted, the cross-examination of Mr Dueppen at T2/115-117 is not relevant to the case on Fabritius. The specific example put to him was a slightly processed oil, i.e. it had undergone only one further processing step, namely a bit of washing to remove some phospholipids (T2/116/14-21). The problem is that the example was incomplete, for there are two possibilities:
i) The slightly processed oil has <55% DHA in TAG. In that case, DSM does not need to rely on the crude oil feature at all. The oil of the invention has a clear and undisputed technical benefit over the slightly processed oil, namely a greater % DHA in TAG.
ii) The slightly processed oil has 55-65% DHA in TAG. But such an oil did not form part of the state of the art. It was impossible to make such an oil before EP740 and therefore DSM is not called upon to distinguish its invention from that oil. Otherwise, DSM submitted, every patent would be invalid if a defendant could posit a thing which was not part of the state of the art and say that the claimed invention had no technical benefit over it.
473. DSM submitted that this is the correct question which has to be addressed.
474. DSM addressed it in two stages.
475. First, DSM relied on the technical benefits of the crude oil of EP740 generally (without, they said, in any way undermining the importance of focussing on the distinctions between claim 1B and Fabritius specifically). These represented a common theme in DSM’s arguments namely that a crude oil, which has not been subject to further processing, has a number of important features. DSM submitted that these points were established in cross-examination [see Wynn XX T4/521-530 and Kyle XX T5/687-696], and I did not detect that Mara was disposed to dispute any of these points (since their point was that all this was irrelevant):
i) A crude oil contains impurities such as free fatty acids, phospholipids, minerals, carotenoids, sterols, antioxidants, waxes and residual cell debris. It contains saturated fatty acids in TAG. It is cloudy at room temperature, as the Single Cell Oils book explains. This was supported by the experts. Such an oil is beneficial in particular because it has the potential to be refined or enriched in a whole range of different ways.
ii) There are a number of different further processing steps (which the skilled person would call RBD or RBWD), and ways of carrying out those processing steps, that may be performed on a crude oil. In general, they involve removing components from the oil. Which of these steps to take, and how, and which components to remove, and how much of them, will depend on the intended use of the end product, customer requirements and so on.
iii) Having a crude oil gives the skilled person all these options. They can, for example, choose to remove as many or as few of the carotenoids as they wish to get the colour of oil that they desire. But once any processing has been done it cannot be undone. The skilled person’s options are therefore limited by any processing that is carried out.
iv) A crude oil is thus “improvable” in a way that a refined oil is not.
v) Therefore, the combination of the oil being a crude oil and high in DHA brings further benefits yet. Having an enriched/refined oil with (say) 60% DHA in TAG would be great. But having a crude oil with 60% DHA in TAG would be even better, because it could be put through standard winterisation to remove non-DHA TAG components, thereby producing an oil with even higher % DHA in TAG. It could also be refined in different ways to produce products that could not be produced from the enriched/refined oil.
vi) Therefore, the higher the percentage of DHA in the crude oil (and in the TAG fraction), the better, as it provides an improved starting point for producing a refined end product. For example, if the skilled person wanted to enrich their oil to hit a yet higher target of DHA, then the more DHA in their crude oil, the easier and more economical it will be to hit that target.
vii) If the skilled person wanted to dilute their oil to have a uniform, lower concentration of DHA in their end product (as Martek did with DHASCO), then the higher the DHA concentration in the crude oil, the more end product they can produce from each litre of crude oil.
476. Second, DSM noted that, as the arguments and evidence developed, there was a focus on:
i) Wynn 4 and XX T5/582-598
ii) Kyle 4 and XX T6/772-803.
477. DSM argued that the Fabritius process does not produce a product which is identical to the crude oil of the invention. At a high level, they said there are two overlapping points to consider:
i) The technical benefits of the crude oil of claim 1B over an oil that could be produced by the Fabritius process.
ii) Whether it would be possible to distinguish the two oils.
478. DSM submitted that the evidence established the following answers to those questions. These submissions were DSM’s answers to Mara’s submissions on Attempts 2 and 3, set out above.
479. First, the Fabritius process removes tri-saturated fatty acid TAGs, such as 16:0/16:0/16:0 (“tripalmitin”) and others (Kyle 1 ¶224):
i) This provides both a means for distinguishing the oils and also a clear technical benefit of the oil of the invention, since it will contain such TAGs, which could be removed to drive the % DHA in TAG yet higher (Wynn 4 ¶¶5, 8).
ii) Dr Wynn was pressed with the possibility of a crude Thraustochytrid oil with very low levels of tri-saturated fatty acid TAGs: he was clear that a Thraustochytrid oil would “inevitably” have above 1% of such species (Wynn XX T5/587/4 - 589/3, especially 588/22-25). That is why both Dr Wynn and Dr Kyle (in Kyle 1 ¶244) chose tripalmitin as an example of a species removed by winterisation/Fabritius.
iii) Then Dr Wynn was pressed with the possibility of a crude Thraustochytrid oil with very high levels of tri-saturated fatty acid TAGs, so that the Fabritius process might not remove them all or virtually all of them. Here, Dr Wynn was clear that his thesis (Wynn 4 ¶5) would apply to every Thraustochytrid oil he had come across and certainly any Thraustochytrid oil with 55-65% DHA in TAG (Wynn XX T5/589/11 - 592/13, especially 591/25 - 592/11). In any event, the tri-saturated fatty acid TAGs have melting points in the region of 60+°C, so they will be efficiently removed by the Fabritius process at -85°C and centrifuging at 0°C. Dr Kyle’s unqualified evidence (supported by EP740 Tables 10 and 14) was that they would generally be removed by normal winterisation (i.e. at around 0°C) and that extreme winterisation at -85°C should remove them all (Kyle 1 ¶127, 4th bullet and XX T6/763/9 - 764/24). (A further point of detail: the attempt in Kyle 4 to rely on Barclay Table 3 or Wynn 1 ¶89 was misconceived, since the data referred to in both cases related to whole organisms, not extracted lipids, and the organisms had not even been grown for TAG accumulation.)
480. Second, the tri-saturated fatty acids are in any event merely an example of components that will be removed. They provide one way in which the oils could be distinguished by a skilled person. The more general point is that numerous components are removed by the Fabritius process for the purpose of increasing the DHA concentration (as confirmed in Kyle XX T6/773/24 - 775/12). DSM said this can be seen from the fact that the examples all result in a loss of around half the starting mass. Those components are gone forever from the Fabritius oil. But they can be removed from the oil of the invention, driving its % DHA in TAG yet higher. This is a clear technical benefit of the latter (see especially Dr Wynn’s explanation of this point at T4/524/10-24).
481. Third, Dr Kyle came up with a further specific example of this general point in the witness box. The “residue” of the Fabritius oil contains significant amounts of DHA. That is likely to comprise monomers of DHA, for as Dr Kyle pointed, out the trimers and dimers of DHA would remain in the oil itself (Kyle XX T6/774/9 - 775/12, see also T6/777/15-17). This provides another means for distinguishing the oils; and another clear technical benefit of the oil of the invention, since it will contain DHA monomers in TAG, which could be removed to drive the % DHA in TAG yet higher.
482. Fourth, a further means of telling the oils apart is the simple cloudiness test. Dr Wynn proposed putting the oils in a fridge. But as the SCO Book makes clear (see [D2/4/60]), normal winterisation turns cloudy crude oil into clear oil at room temperature. So extreme winterisation will certainly have that effect. (The concept of a non-cloudy crude oil was yet more unfounded speculation from Dr Kyle (Kyle XX T6/796/2-6)).
483. Fifth, the Fabritius process also increases the concentration of other PUFAs which may or may not be desirable (see Kyle 1 ¶¶66 and 174, Kyle 2 ¶17) (Dr Kyle claimed repeatedly that the ratios remained the same, but simple maths performed on the Fabritius examples showed him to be wrong.)
484. It is convenient to address the issues in the same order as the parties did and to start by considering the appropriate characterisation of claim 1B.
485. For this purpose, I found it helpful to recast claim 1B (and, in this regard, I ignore the issues around ‘from at least 55% to 65%’). It is, in effect a claim to a microbial oil with two particular features or characteristics: first, DHA in TAG of 55-65% and second, 5% or less of HDA obtained by:
i) No further processing after extraction from the biomass
ii) Of a thraustochytrid microorganism.
486. As Birss J. stated at [147(ii)] of Hospira, for the purposes of novelty, the claim is treated as being ‘obtainable by’. I cannot see any reason why the same approach should not apply for the purposes of obviousness, following the logic of Birss J. in [145], and every reason why it should. This is a short point of law. In this regard, I note that DSM made no attempt to grapple with this point explicitly but, as I have indicated, they took a different approach.
487. I will now assess whether DSM’s different approach provided or included any reason not to apply this logic.
488. It is necessary to unpick DSM’s arguments which were made on several different levels.
489. First, I was looking for the legal basis for DSM’s asserted relevance of their ‘technical effect/benefit’ argument. I have set out their contentions on the law of ‘technical effect’ above but they did not seem to me to engage with any of the issues regarding the process elements of claim 1B, as explained by Birss J. in Hospira.
490. Despite the detail in DSM’s argument, in my view it suffers from a fundamental flaw. DSM’s whole ‘technical effect’ argument appears to me to have been founded on Mara’s pleading that the ‘crude oil…without further processing’ element has no technical effect. It can be seen from [463] above that Mara did take this point, but it was a standalone and secondary submission. However, the fact that Mara pleaded and pursued this point does not make it the critical nor the correct question so far as the correct characterisation of claim 1B is concerned.
491. For this simple reason, when it comes to the question of the correct characterisation of claim 1B and its process elements, the whole of DSM’s argument on technical effect seemed to me to be an irrelevant distraction. The only part which has any relevance at all is DSM’s contention that it is possible to distinguish between a crude oil of claim 1B and an oil which has passed through Fabritius Example 2. However, as Mara submitted, even this contention is aimed at the wrong question. The correct question is whether the purported limitation is a feature of any oil at all. In my view, DSM wholly failed to persuade me that the process elements conferred any product characteristic, for the reasons submitted by Mara, as set out above.
492. Furthermore, I reached the clear conclusion that DSM’s Attempts 2 and 3 addressed the wrong question. The cross-examination sought to demonstrate that the crude oil of claim 1B of EP740 was not identical to an oil with the product features of claim 1B which had passed through the processing in Fabritius Example 2. However, even if there were differences in tripalmitin or any of the other fatty acids referred to in DSM’s argument, none of those fatty acids feature in claim 1B. Accordingly, they cannot provide any point of distinction. Thus, asking whether the oils were identical i.e. in every particular was the wrong question.
493. In any event, I was left unpersuaded by Dr Wynn’s evidence that his means of distinguishing the oils would be applicable to all Thraustochytrid oils, bearing in mind that, as I understand matters, only a small subset of those oils have actually been extracted and characterised.
494. It is convenient at this point to turn to consider Mara’s objections to the amendments to reach claims 1B and 2B. As I indicated above, these arguments reinforce my conclusions as to the correct characterisation of claim 1B.
495. Mara’s position on the amendments was that, as well as failing to cure invalidity, they should not be permitted as not being clear and concise (for several reasons) but also because they add matter (which I address in a separate section below).
496. Section 14(5) requires that the claims should be clear and concise. On these requirements, Mara drew my attention to the following caselaw and principles.
497. Section 14(5) was considered by Sales J in Teva UK Limited v AstraZeneca AB [2014] EWHC 2873 (Pat) [JA2/23/439]:
‘109. A claim in a patent does not need to be drafted to a standard which removes all conceivable doubt about what it means, but it “needs to be as clear as the subject matter reasonably admits of”: LG Philips LCD Co. Ltd v Tatung (UK) Ltd, at [20] per Neuberger LJ; see also [26]. Absolute clarity would impose an unreasonable standard and would undermine fair protection for inventions under the patent system. On the other hand, a patent defines an intellectual property right and the scope of the patentee's legally protected monopoly, and potential competitors are entitled to fair warning and a reasonable indication from the face of the patent what its scope is.’
498. The general prohibition on framing a claim as a product by process, except under very limited circumstances, is also treated as falling under this head. Lord Hoffmann explained the prohibition at [91] of Kirin-Amgen [JA2/13/21]:
‘The only case in which the EPO will accept a claim to a product defined in terms of its process of manufacture is when the product is new in the sense of being different from any existing product in the state of the art but the difference cannot be described in chemical or physical terms. As the Board said in International Flavors (at paragraph 8):
"This may well be the only way to define certain natural products or macromolecular materials of unidentified or complex composition which have not yet been defined structurally."’
499. Likewise, in Hospira v Genentech, Birss J explained at [135] that [JA1/11/537]:
‘The EPO's approach to overt product by process claims today is settled. They will be permitted (and only permitted) if there is no other way of defining the product open to the patentee. This is a decision based on policy. Such claims present clarity problems and are best avoided but if there is no alternative way of defining the characteristic in question, then they will be permitted.’
500. This is reflected in the EPO’s Guidelines for Examination, Part F, Chapter IV, Section 4.12 (Clarity and Interpretation, Product-by-process claim) [JA2/27/549]:
‘Claims for products defined in terms of a process of manufacture are allowable only if the products as such fulfil the requirements for patentability, i.e. inter alia that they are new and inventive, and it is impossible to define the claimed product other than in terms of a process of manufacture. A product is not rendered novel merely by the fact that it is produced by means of a new process.’
501. To similar effect is the following statement in the Case Law of the Boards of Appeal, 10th ed., II.A.7.3: “The criterion laid down in T 150/82 (OJ 1984, 309), whereby it must be impossible to define the claimed product other than in terms of a process of manufacture is now established case law…” .
502. Section 7.1 explains that a consequence is:
‘With regard to product-by-process claims, the requirement of clarity means that the skilled person should be able to determine, either from the claim alone or, by construction of the claim in the light of the description, or by construction in the light of the skilled person's common general knowledge, which identifiable and unambiguous technical features are imparted to the product by the process by which it is defined (T 967/10, T 1988/12, T 354/17, T 2243/18).’
503. In other words, the skilled person must be able to identify unambiguously what product features are imparted to the product by the process step. Mara pointed out that DSM accepted this principle in their Opening Skeleton at [47], but DSM went on to submit that this does not mean that the claim or patent has to specify expressly every technical feature of the product. DSM’s reason was because “that is impossible in the case of every product by process claim. Nor do the technical features have to be unique to the process in question. Novelty is still to be judged by reference to the whole claim”. However, just because it may be impossible for some product by process claims does not mean it is impossible for claims 1B and 2B.
504. Mara submitted that this is a strict test - it is not enough to know in a general way that the product has some property. The skilled person has to be able to nail down what it is. Mara referred to T 967/10 (cited in section 7.1), where the claim was to a lettuce plant. It defined the plant as being the product of crossing a plant that was susceptible to a particular pathogen, with one of a defined set of resistant plants, and selecting for resistant progeny. The TBA held that the skilled person would understand that the process feature implied the presence in the genome of the claimed plant of genetic information identical to that present in the genome of the resistant strain that had been used to produce it (¶7). But that did not tell the skilled what that genetic information was, and they had no way of knowing. The claim therefore lacked clarity, since the identifiable and unambiguous technical features of the claimed product could not be determined (¶¶8-11).
505. The same approach is adopted in the UK. The Manual of Patent Practice states at 14.120.1 that "A claim for a patentable product defined by its process of manufacture is only allowable if the product cannot satisfactorily be characterised by reference to its structure or composition; if the product can be defined by other means, an objection under clarity and/or conciseness should be raised” [JA2/28/550].
506. Therefore, as Mara submitted, the law and practice are the same in the UK and the EPO, but in any event s.75(5) requires that in considering whether or not to allow an amendment, the court shall have regard to any relevant principles applicable under the EPC. Birss J decided in Hospira v Genentech at [142] that s.75(5) means that the court should follow the principles applied by the EPO in the context of considering whether to permit an amendment to create an overt product by process claim [JA1/11/538].
507. I did not understand DSM to dispute any of these principles. Indeed, in their Opening Skeleton DSM expressly accepted the principles I covered in [501] and [503] above.
508. Mara contended that claim 1B is not clear and concise, contrary to section 14(5)(b), for the following reasons:
i) First, the feature “from at least 55% to 65%” in claim 1B is not clear or concise on its face (and similarly for “from at least 60% to 65%” in claim 2B).
ii) Secondly, the amendments illegitimately use “product by process” form in two respects: that the oil is “extracted from … a thraustochytrid microorganism”, and that it is “a crude oil extracted … without further processing” (a process feature specified by the absence of steps being is as much a process feature as one specified by their presence).
iii) Thirdly, it would be unclear to the skilled person what the difference would be between an oil derived from a thraustochytrid microorganism and an oil derived from another oil-producing microorganism, and/or it would be unclear which identifiable and unambiguous technical features are imparted to the oil by being derived from a thraustochytrid microorganism or by being a “crude oil… without further processing”.
509. I will address these in turn, even though the first is relatively inconsequential.
510. On this objection, Mara submitted in opening that it is simply unclear what it means to specify the DHA percentage as being “from at least 55% to 65%”. Mara posed a series of rhetorical questions: does this mean a range “from (at least 55%) to (65%)” or is it specifying a minimum that lies within a range, in the sense “from (at least 55% to 65%)”. Is this wording said to differ from “from 55% to 65%”? If it does differ, how? If it does not differ, the words “at least” are both unclear and otiose, so the claim lacks conciseness. The subject matter reasonably admits of greater clarity than provided by this amendment, so it falls foul of s.14(5).
511. DSM contended that this expression can only be read as defining a range for the % DHA in TAG of 55% to 65% (in claim 1B) and 60% to 65% (in claim 2B). DSM acknowledged that the words “at least” are unnecessary but submitted they do not cause any real difficulty in understanding what the patentee means.
512. DSM also explained that the words “at least” remain in the amended claims for consistency with the amendments proposed in the parallel opposition proceedings in the EPO. Those acting for DSM in the EPO are following the conventional practice of making the minimum necessary amendments, rather than seeking to draft the perfect claim. Finally, DSM submitted that if there was any merit in this objection, it could be overcome by deleting the words “at least”.
513. In closing, Mara dropped this objection, evidently because they thought that keeping the words ‘at least’ strengthened their hand on added matter. However, I find this is a valid objection, for the reasons set out by Mara. DSM’s arguments as to consistency and practice in the EPO have to give way to the statutory requirements. However, I agree that the objection is overcome by the deletion of the words ‘at least’.
514. Mara’s point was that this is not any kind of limitation that relates to a real characteristic of the oil at all and that this is in reality no more than a description of the history of the process by which the oil has been obtained - i.e. the phylogenetic order of the cell that was used in the process.
515. Mara submitted it is not a characteristic of the oil itself, and, further, that the skilled person would not know what the technical features are of the claimed oil that arise from it having been derived from a thraustochytrid organism. They pointed out that no specific characteristic has been suggested.
516. Whilst accepting the principles (as I mentioned in [507]), DSM’s primary position was that the thraustochytrid integer did not introduce a process element at all, submitting that the claim says nothing about the process by which the microbial oil is to be extracted from the Thraustochytrid. Simply specifying the ingredients of a product does not create a product by process claim. I reject this. The starting point of the process is nonetheless part of the process.
517. DSM’s secondary position was that this was a paradigm case of a permissible product by process claim because it would be impossible to frame a sensible, concise claim, which sufficiently characterised an oil derived from a Thraustochytrid, without using the word “Thraustochytrid”. In support, they suggested the reference to thraustochytrids is just specifying ‘ingredients of a product’ and argued it was akin to specifying ‘olive oil’.
518. DSM’s suggested analogy was that olive oil was plainly different to sunflower oil. However, as Mara submitted, DSM’s example referred to two specific oils from specific individual species of plant (Olea europaea and Helianthus annuus respectively) that produce oils with different make up. In that example, it means something to speak about olive oil, as opposed to sunflower oil.
519. Mara submitted that claim 1B is a claim not to oil from an individual species - for example the PTA-9695 new strain that is actually DSM’s contribution. It is not even a claim to all oils from organisms in the genus to which that species belongs. Nor even is it limited to the next level up of the taxonomy - the family to which the species belongs. It specifies the entire order.
520. As to this Dr Wynn’ evidence (T5/593/5-16) was (with emphasis added):
Q. He points out there are a vast number of Thraustochytrids with
a wide range of fatty acid profiles. He is right about that; yes?
A. Absolutely; yes.
Q. It is plain both in 2009 and today that there will be many
Thraustochytrids not even identified yet; yes?
A. Absolutely; yes.
Q. Even at 2009 on those identified, there was no pattern about
the fatty acid profile; agreed?
A. No patent about the fatty acid profile?
Q. No "pattern" that had emerged?
A. Not that I am aware of.
521. The key point in that exchange is also confirmed in [0006] of EP740 where it says: ‘…isolated thraustochytrids vary in the identity and amounts of LC-PUFAs produced..’
522. DSM made a variety of other submissions by way of alternative secondary positions and I will attempt to cover all of them.
523. DSM’s opening skeleton proposed at ¶¶49-50 that the relevant technical feature of being derived from a thraustochytrid is “presence of high levels of DHA”. But that is the subject of the DHA integer, and of course the level of DHA in thraustochytrids is very variable - Dr Wynn made the point that until EP740 nobody had been able to find one with a DHA level as high as PTA-9695. So I agree that that cannot be the identifiable and unambiguous technical feature imparted by being a thraustochytrid.
524. DSM then suggested that containing DPA is a distinguishing feature of thraustochytrid oils (as compared to C.cohnii, which produced DHA but not DPA) (skeleton ¶50). But no cut off level of DPA has been or could be suggested, and thraustochytrids had differing levels of DPA. On this point, Mara sought to rely on Barclay Table 3, column headed C22:5w6, which shows widely varying levels of DPA from 0.0% (which Dr Wynn appeared to contemplate in one of his answers) to more than 20%. DSM objected on the basis that this was not the subject of any evidence from any expert, but that was incorrect. Although Mara dropped reliance on Barclay as prior art in the course of the trial, the expert evidence which was led still discussed Barclay. In any event, I agree that this cannot be the necessary identifiable and unambiguous property of a thraustochytrid oil. Furthermore, how is the skilled person supposed to know that the comparison should be to C.cohnii?
525. DSM’s oral opening had yet another suggestion, that it was simply the fatty acid profiles of the thraustochytrid oils that constituted the required identifiable and unambiguous technical feature [T1/34/8-13]. Mara submitted that this was simply to re-state the problem, not to answer it. What is it about the fatty acid profile that characterises the claimed products? I agree that a mere reference to the or a ‘fatty acid profile’ is exactly the sort of vagueness that the EPO says cannot satisfy the requirement (see [502] above).
526. In closing argument Ms Nezami made the submissions for DSM on this part of the case and she submitted that the narrowing of the claim to a thraustochytrid oil defined a feature of the product itself, the feature being the fatty acid profile of a Thraustochytrid microorganism. My response to this submission was to ask what that fatty acid profile was - in other words, what should I write down. The answer was nebulous - that Thraustochytrids produce a fatty acid profile which is a source of high DHA and comprises DPA. It was also said that it is not possible to give the full detail of the fatty acid profile - and that was said to justify the inclusion of the process feature. I then asked how did I know that all Thraustochytrids will give a high DHA level to which the response was ‘That is the evidence’. This was a bold submission bearing in mind the evidence was to precisely the opposite effect.
527. I did not find any of this reasoning at all persuasive. EP740 sets out the fatty acid profile of samples A1 and A2 of the crude oils extracted from ATCC No.PTA-9695, and (taking due account of the experimental variability one might expect) they are remarkably similar. This shows that it is perfectly possible to define (at a suitable level of generality) the fatty acid profile for the crude oil extracted from a given strain of thraustochytrid, provided the oil has actually been extracted and analysed. However, DSM’s inability to define a suitable fatty acid profile or profiles or even ranges of the entire thraustochytrid order stems from the fact they have only extracted and analysed the oils from the PTA-9695 strain and some mutants derived therefrom, and not from any inherent impossibility to do so.
528. On this point, the fact that DSM have now slightly limited the original vastly broad claim 1 to cover the entire thraustochytrid order cannot, in my view, be used to excuse their failure to designate relevant characteristics of the oils which they seek to claim.
529. The upshot is that the evidence I quoted above elicited from Dr Wynn is fatal to any suggestion that referring to an oil obtained from a thraustochytrid is in any way referring to a characteristic of the oil itself, as it may be for e.g. an olive oil.
“crude oil… without further processing”
530. That third objection also applies in respect of the “crude oil… without further processing” feature.
531. For the most part, DSM appeared to ignore the problem altogether, and addressed what one might call ‘conventional’ or ‘literal’ clarity contending that nobody is actually contending there is any lack of clarity in these words. This did not address the product by process issue at all.
532. DSM also said ‘We cannot think how a crude oil product could possibly be defined other than by saying it is a crude oil that has not undergone those [further processing] steps.’ But this proves Mara’s point that this is a process feature and not a feature of the product.
533. As I discussed above, DSM made multiple attempts to come up with a basis for arguing that the limitation relates to a characteristic of the oil itself rather than a process limitation: see [422] et seq. above.
534. The additional point made by Mara was that, even if DSM managed to persuade the Court that any of their Attempts 1 to 3 did mean that this is actually a feature of the product, the very fact that it has taken DSM so many attempts shows that the skilled person at the priority date would have been completely unclear as to what “identifiable and unambiguous technical features” the claim feature imparted. Mara pointed to the multiple attempts by an expert (and counsel) to come up with alternative suggestions as to what the product feature really is. The poor skilled person would have had no idea. I agree.
535. To conclude, (i) the claim does not allow the skilled person to understand what the characteristics of the claimed oil are, as a product, and (ii) it was and is possible to define the claimed oil(s) by way of product characteristics e.g. by way of a fatty acid profile. Accordingly, the claim is unclear as a result of the EPO requirement set out at [502] above.
536. Accordingly, DSM’s application to amend must be dismissed.
537. Mara pleaded insufficiency in a variety of ways (not least because there was a wider target than just claims 1B and 2B at that stage). At trial, the allegations of insufficiency which were pursued by Mara were:
i) The claims are not enabled across their scope.
ii) The claim is too broad because the inventive contribution lies in the identification of a particular strain designated ATCC PTA-9695.
538. At first sight, it appeared that Mara took a conventional approach to these allegations. By contrast, DSM’s argument was that all oils from any thraustochytrid are of equal value and utility. That was then deployed to argue that the breadth of the claim is not a relevant range across which the claim needs to be enabled. These contrasting positions engaged different principles from the authorities, to which I now turn.
539. Mara explained that their insufficiency case engaged the basic tenet that the extent of a patent monopoly should correspond to, and be justified by, the actual technical contribution to the art, relying on Lord Hodge in Actavis Group PTC EHF v ICOS Corporation [2019] UKSC 15 at [57]:
The general principle that the extent of the patent monopoly should correspond to and be justified by the actual technical contribution to the art is thus part of the jurisprudence of both the EPO and the UK courts and, as Lord Sumption observed in Generics v Warner-Lambert (above), para 17, “the principal conditions of validity, novelty, inventive step, industrial application and sufficiency are all, in one way or another, directed to satisfying the principle thus expressed”.
540. One consequence of this basic tenet is that a patent is insufficient (in the Biogen sense) if it extends to embodiments which owe nothing to the patentee’s contribution to the art.
541. In Biogen the trial judge had found that Professor Murray’s method was capable of making both the hepatitis B core antigen (HBcAg) and its surface antigen (HBsAg). This was a finding that the House of Lords held should stand (Biogen Inc. v. Medeva Plc [1997] RPC 1 at 50). The patentee had therefore enabled routes to the claimed invention. Lord Hoffmann went on to explain, however, that this was not enough:
‘But the fact that the skilled man following the teaching of Biogen 1 would have been able to make HBcAg and HBsAg in bacterial cells, or indeed in any cells, does not conclude the matter. I think that in concentrating upon the question of whether Professor Murray's invention could, so to speak, deliver the goods across the full width of the patent or priority document, the courts and the E.P.O. allowed their attention to be diverted from what seems to me in this particular case the critical issue. It is not whether the claimed invention could deliver the goods, but whether the claims cover other ways in which they might be delivered: ways which owe nothing to the teaching of the patent or any principle which it disclosed.’
542. Mara also relied on the review of the case law on Biogen insufficiency by Floyd LJ in Anan Kasei Co. Ltd v Neo Chemicals and Oxides Ltd [2019] EWCA Civ 1646 and relied on his summary of the principles at [52]:
‘52. I draw the following from the speeches in these two cases:
1. The principle in Biogen is concerned with permissible scope of claim in the light of the patentee's contribution to the art.
2. In general, that principle is that the claim must not extend to embodiments which owe nothing to the patentee's contribution to the art.
3. In the case of a claim to a single novel chemical compound, the patentee's technical contribution is that compound. Such a claim will not be insufficient if the single compound is enabled by a method in the specification, notwithstanding the fact that there may be other methods of making it which owe nothing to the disclosed method.
4. The same must be true of a claim to a class of compounds, each of which can be made by the application of a method disclosed in the specification. There is no requirement that the patentee disclose more than one method, where one method will do.
5. This does not mean that all claims to a class of products by definition comply with the Biogen principle. The conclusion in Biogen shows that a claim which is formally to a class of products may cover embodiments which owe nothing to the patentee's technical contribution.
6. The reason why the claim in Biogen offended the principle was not because it had "process components" but because the language of the claim was so generalised (both in relation to the manner in which the product was made and in relation to its function) that it extended to embodiments which owed nothing to the patentee's contribution to the art. A claim to a product defined by its function (e.g. any heavier than air flying machine referred to by Lord Hoffmann at page 52 in Biogen) is capable of extending to subject matter which owes nothing to the patentee's contribution to the art.’
543. Floyd LJ went on to point out that claims defined by reference to desired properties of a product need to be scrutinised carefully for reasons explained by Jacob LJ in Generics v Lundbeck [2008] EWCA Civ 311, [2008] RPC 19 at [60]-[62]. He had referred to the EPO’s approach that claims which attempt to define the invention by a result to be achieved should not be allowed, in particular if they only amount to claiming the underlying technical problem. He explained the consequence in this way:
‘61. So, for example, if a man finds a particular way of making a new substance which is 10 times harder than diamond, he cannot just claim "a substance which is 10 times harder than diamond." He can claim his particular method and he can claim the actual new substance produced by his method, either by specifying its composition and structure or, if that cannot be done, by reference to the method (see Kirin-Amgen at [90-91]) but no more. The reason he cannot claim more is that he has not enabled more - he has claimed the entire class of products which have the known desirable properties yet he has only enabled one member of that class.’
544. Floyd LJ reiterated at [55] that the underlying rule is that the patentee cannot claim more than he has enabled. Whether he has claimed more than he has enabled is a question of fact which falls for decision on the evidence in the case.
545. As to the law on insufficiency more generally, including undue burden, Mara drew my attention to Kitchin LJ’s classic exposition in Regeneron v Genentech [2013] EWCA Civ 93, [2013] RPC 28 at [95]-[103]. Birss LJ expanded upon the interplay between a functional limitation and undue burden in FibroGen v Akebia [2021] EWCA Civ 1279, [2022] RPC 7 at [51]-[97].
546. DSM were critical of Mara for not having cited Regeneron v Kymab [2020] UKSC 27, [2020] RPC 22 and in particular Lord Briggs’ summary of the principles at [56], and drew attention to the pertinent sub-paragraphs. DSM contended that three legal principles were relevant. First, the principle that where the invention is a new product, which the patentee has made available for the first time, they are entitled to claim the product even if there are other ways of making it, relying on Generics v Lundbeck [2008] EWCA Civ 311, [2008] RPC 19, [36] (Lord Hoffman), [52] (Jacob LJ); upheld [2009] UKHL 12, [2009] RPC 13, [81]-[83] (Lord Neuberger). This is also Regeneron v Kymab point (ii). In Lundbeck, the technical contribution was the new product (the isolated +enantiomer of citalopram), which the patentee had enabled for the first time, and the patentee was therefore entitled to claim that new product.
547. Second, in the case of a product claim to a range of products, the patentee has to enable substantially all products falling within the claim (not all ways of making those products). (Regeneron v Kymab, point (iv)). See also Anan Kasei v Neo Chemicals [2019] EWCA Civ 1646, [52.4] (Floyd LJ), emphasising that the patent does not need to enable all ways of making those products.
548. But, third, that is subject to principle (vii) of Lord Briggs' summary of the relevant legal principles in Regeneron v Kymab at [56](vii):
‘… The requirement to show enablement across the whole scope of the claim applies only across a relevant range. Put broadly, the range will be relevant if it is denominated by reference to a variable which significantly affects the value or utility of the product in achieving the purpose for which it is to be made.’
549. DSM submitted that Birss J gave useful guidance on the application of this point in Illumina v Latvia MGI [2021] EWHC 57 (Pat), [2021] RPC 12, [276]-[279], including this:
‘iv) An example of another range, not relevant in the Regeneron sense, will be a descriptive feature in a claim (whether structural or functional) which can cover a variety of things, but for which that variety does not significantly affect the value or utility of the claimed product or process in achieving its relevant purpose. The relevant purpose is judged in all the circumstances, starting from the terms of the claim itself but also, where appropriate, by reference to the essence or core of the invention.
v) For a claim feature which amounts to a range in this other sense, the skilled person must still be able to make a suitable selection, without undue burden, in order for the claim to be sufficiently disclosed. However provided that is so at the relevant date, such a claim feature will not be insufficient simply because it is capable of also covering within its scope things which had not been invented at that relevant date.’
550. DSM submitted that this is the correct analytical approach. It gives effect to the general principle that the extent of the patent monopoly should be justified by the actual technical contribution to the art. But note that principle is the “underlying purpose” of the statutory requirements of novelty, sufficiency, etc (as explained in Generics v Warner-Lambert [2018] UKSC 56, [2018] RPC 21, [17] (Lord Sumption)); or to put the point the other way round, sufficiency is one of the tools by which that principle is given effect (Regeneron v Kymab at [23] & [56]). It is not a separate, free-standing point, requiring the Court to make a subjective evaluation without any analytical framework. Such a free-standing evaluation was, so DSM submitted, perhaps and with respect, the error made by Kitchin J as he then was in Generics v Lundbeck.
551. DSM argued that it therefore remains essential for a party attacking a patent on this ground to identify something relevant falling within the claim that is not enabled. They contended that the point is well-illustrated by Anan Kasei v Neo Chemicals in the judgment of Floyd LJ:
‘59. To establish that the claim offended against the Biogen principle as explained in Lundbeck, however, [the defendants] had to go further. They had positively to establish that there were structures which were covered by the claim which could not be made with the benefit of that teaching. There is no reason for the court to assume that the claim covers structures which owe nothing to [the patentee’s] contribution to the art.’
552. The Court went on to say at [60] that even though there were other methods of manufacture, that was not enough. It had to be established that such methods produced products within the claim (subject, now, to Regeneron v Kymab point (vii)) and that they were incapable of being replicated by the patentee’s method, or a suitable CGK adjustment to it. The attack on the patent failed because the defendants had not established these matters in evidence.
553. Consistently with their citation of Regeneron point (vii), in their oral opening, DSM criticised Mara for not referring to the decision of the Supreme Court in Regeneron. This highlighted a key issue between the parties as to whether this case involves a relevant range in the Regeneron sense. To resolve that it is necessary to turn to the facts.
554. Mara’s position was that if there is any contribution at all in EP740 it is the provision of a new strain - PTA-9695 - that can be used to make a DHA-rich oil in accordance with claim 1B. For claim 2B, Mara contended that even that strain does not have levels in accordance with the claim when extracted with hexane (58.1% DHA as a proportion of triglycerides), but just scrapes in by FRIOLEX extraction (60.4%).
555. Other than strain PTA-9695 in Example 3, Mara submitted there is no teaching in EP740 of how to produce a microbial oil having the features of claim 1B. None of the other strains or mutants are disclosed as having the features of claim 1B - the levels of DHA in the triglyceride fraction are not measured, nor are the levels of triglycerides in the crude oil as a whole.
556. Consequently, the only way to arrive at an oil having the features claimed in claim 1B (or 2B) that is taught in EP740 is to use the PTA-9695 strain disclosed, applying the culture and extraction conditions described in Example 3.
557. As Dr Kyle explained (Kyle 1 ¶308), the only thing of value the skilled person would learn from EP740 is that the patentee had found and deposited one notable strain - PTA-9695 - which would be useful for making high-DHA oils (potentially along with mutants of it, although not all of these are said to have been deposited and thus made available to third parties). Otherwise, EP740 does not help the skilled person produce oils falling within the scope of the claims or add to the skilled person’s existing bank of knowledge beyond what they would already know from their CGK and/or the prior art. The skilled person could purify existing oils based on their CGK or could go out and screen for more strains in the manner well known to have been done by OmegaTech (and reported in the Barclay patent). But EP740 does not help with this at all.
558. As shown by the wide variety of fatty acid profiles exhibited by prior art microbial strains, this is a field in which the identity of the strain is crucially important to the properties of the resulting oil. The microbes function as biological oil factories, and each factory makes a different oil with different levels of DHA and other fatty acids. This is not a property that is defined by a taxonomic order or genus, but by individual strains. For example, even though it was known as a matter of CGK that some thraustochytrids had potential for being producers of DHA, this information in itself did nothing to reduce the hard graft of finding the particular strains that had particularly desirable properties. Mara pointed to Table 3 in Barclay, where the strains showed widely divergent fatty acid profiles.
559. Dr Wynn’s evidence was that finding good strains was incredibly hard. Referring to the situation at the priority date of EP740, he said that extensive searches had failed to find a strain that improved upon Barclay’s thraustochytrid strain ATCC 20888. See Wynn 1 ¶276:
‘Many new research teams had implemented the Barclay method to attempt to identify and isolate new and improved strains, whilst some teams had identified strains with different ratios of omega-3 fatty acids, the Skilled Microbiologist would not be aware of any team that had isolated a strain that materially outperformed the production strain (ATCC 20888) in relation to DHA levels, despite having been trying for around 17 years.’
560. This reflected Dr Wynn’s evidence on CGK at the EP740 priority date, in which he referred to the considerable academic and commercial interest in thraustochytrids in light of Barclay’s DHA oil, with one of the research focusses being on the isolation of new strains to produce new SCOs (Wynn 1 ¶106), and the failure, despite considerable research and the identification of a wide range of new strains, to find one that was a better DHA producer than ATC 20888 (¶107).
561. Against that background, it is not suggested that EP740 does anything more than report a particular, single new strain identified using CGK techniques. Mara therefore submitted that its contribution is therefore plainly limited to that strain (and the oil produced from that strain). It has not contributed any other strain, or any other oil. Yet it lays claim to a whole swathe of the field of high DHA oils on the strength of that one strain, while leaving untouched the burden on the skilled person to screen for further strains that produce oils within the claim. Mara contended that this is an archetypal example of a claim that exceeds its technical contribution, and it is insufficient. To the extent the claim covers oils produced from strains other than PTA-9695, the claim imposes a burden that is plainly undue.
562. In closing, DSM set out a series of reasons why they contended that Mara’s case was wrong. These reasons were a slight restatement of what DSM said in opening.
563. DSM contended first, that the invention is a novel and non-obvious oil having the characteristics of the amended claims, in particular having more than 55% DHA in TAG. The patent both demonstrated that it was possible to make such an oil and enabled such an oil to be made for the first time.
564. In DSM’s second point, they acknowledged that the term “thraustochytrid” in claim 1B covered a range of strains, but they contended it is not a relevant range in a Regeneron sense, on the following basis. First, they contended that the strain used does not significantly affect the value or utility of the product in achieving the purpose for which it is to be made. Second, that the value and utility of the claimed microbial oil is the high quantities of DHA in a crude oil extracted from a Thraustochytrid biomass without further processing. Third, DSM acknowledged that the particular strain has to be suitable in the sense that it produces high quantities of DHA, but the particular (suitable) strain does not affect the value or utility of the product. Therefore, it is said, it is not necessary to identify every suitable Thraustochytrid strain that can make the microbial oil of claim 1B.
565. In passing I mention that, in this regard, DSM relied on some evidence from Dr Kyle, in which they contended he said that it is irrelevant that the microorganism of the claim is a Thraustochytrid. DSM presented this evidence as agreement that it was irrelevant which strain within the Thraustochytrid order is used.
566. In fact, what Dr Kyle said in his first report at [274] was that
‘… I do not believe that the skilled person would have considered the identity of the microbe from which the oil is produced to make any technical difference and I believe the Skilled Person would have considered this feature of the claim to be somewhat arbitrary. The Skilled Person would have been concerned with the composition of the resulting oil and not the identity of the microbe from which it was produced.’
567. Instead of supporting DSM’s position, in my view this evidence rather supported Mara’s point that the thraustochytrid limitation did not relate to any characteristic of the product.
568. DSM’s third point was that Mara had failed to establish that there were products falling within the claim that could not be made using EP740’s teaching together with the CGK. It was essential, so DSM said, for Mara to identify such an alleged product, not least so as to give DSM a chance to establish that (a) the product was not within the claim and/or (b) it was not a relevant range and/or (c) it was in fact enabled by the specification with the CGK. DSM contended that Mara’s claim therefore fails for the same reason the sufficiency attack failed in Anan Kasei v Neo Chemicals, above.
569. Dr Wynn’s written evidence suggested that choosing thraustochytrids provided processing benefits - see Wynn 2 ¶69 referring to the benefits identified at Wynn 1 ¶103. Those ¶103 processing benefits were: the ability to grow the cells (i) in low salinity, (ii) in low oxygen and (iii) at higher densities. Dr Wynn’s written evidence accepted that the DHA and TAG levels were ‘commercially desirable features of the oil itself’ (emphasis added, and confirmed at [T4/519/13-20]) - see Wynn 2 ¶70. But he went on to explain that his view was that the value of these levels being provided in a crude oil was that the processes known to manipulate the properties of the oil through refinement are not necessary - see Wynn 2 ¶72.
570. Under cross-examination Dr Wynn accepted that the processing benefits he was referring to in ¶103 were benefits to the manufacturer of the oil because they allow easier processing - see [T4/520/22 - 521/3], particularly at 521/2-3:
Q. They are not actually a property of the oil itself agreed?
A. I do agree yes.
571. Dr Wynn also agreed he had the distinction in mind as to something that was a property of the oil itself when framing his ¶70 (see [T4/521/4-15]). The significance of that is the only thing he identified as a property of the oil itself was the commercially desirable features mentioned in ¶70.
572. As for the feature of the claim as to the oil being a crude oil and not subject to further processing, Dr Wynn’s point in his written evidence was, as mentioned above, that the oil with the high levels of DHA can be obtained without the need for downstream processing steps - he repeated that in XX at [T4/522/4-10].
573. Dr Wynn further accepted this characterisation of his point [T4/523/22 - 524/3]:
Q. Am I fair in characterising your point thus: you have a way
to get an oil with the commercially desirable features that
you identify, its high TAG content and high DHA content,
without having to undertake those refinement and enrichment
processes?
A. That is true, yes.
574. In XX Dr Wynn went on to try to suggest a different point not in his written evidence: that having the crude oil allows one to process in ways that one could not ‘with an oil less than 55% in’. [T4/524/7-8]. This whole passage which extends through to [T4/530/24] was somewhat confused. I agree with Mara that it (a) was not in his written evidence in Wynn 1-3 and seemed to be motivated by a desire to get in evidence in Wynn 4 (see [T4/524/23-24] - at this point Dr Wynn had listened to the argument about its admission but he knew I had not ruled on it).
575. What Dr Wynn sought to suggest was that if processing had been carried out on an oil, it could not be done again. It transpired that what he really had in mind was simply winterisation, not other processing steps.
576. Mara submitted his point was incomprehensible because one can obviously take both a refined oil and a crude oil forward in any way one wishes. If a crude oil with lower DHA content is processed so that the DHA content is increased and the saturated content reduced, it can be processed in the same way as an oil with those enhanced levels of DHA and lower levels of saturates that was obtained straight from extraction - i.e. a crude oil.
577. The upshot of Dr Wynn’s cross examination was that the only features of the claim that are a benefit as regards the oil itself are the commercially desirable features - the DHA and TAG levels - achievable in a processed oil. Everything else is a processing benefit. And moreover all DSM has contributed is one strain which can be used to enjoy those processing benefits.
578. Even assuming the limitation that the oil is extracted from a thraustochytrid cell is a proper limitation as to the oil rather than its history (as to which see above), Mara submitted that the breadth of the claim in that dimension is vast. All EP740 enables is one individual species to be used - see above.
579. Mara submitted that DSM did not suggest this is wrong, nor could it, given Dr Wynn’s evidence as to how difficult finding new strains was - his ¶276 (first report), which I quoted at [559] above. Instead, as revealed in their Opening Skeleton, DSM took a very different approach. As already indicated, DSM argued that all oils from any thraustochytrid are of equal value and utility. That assertion was then deployed to argue that the vast breadth of the claim in not a relevant range across which the claim needs to be enabled.
580. However, it is clear that this argument was deployed without any basis in the evidence. As Mara submitted, it is, in any event, unsustainable after the cross-examination of Dr Wynn who accepted the following points:
i) That there was a vast range of thraustochytrids with varying fatty acid profiles; and that no pattern had emerged as to that (see transcript extract quoted at [520] above). The enormity of the range covered by the whole order was also established at Wynn XX [T5/566 3-24];
ii) The make up as to how much is TAG and how much is other components would vary widely - specifically the levels of the other components vary significantly: see [T5/567/6 - 568/10];
iii) As for TAG specifically, higher TAG levels are generally better - agreed by Wynn [T5/568/11 - 569/5]. Accordingly, it cannot be said all thraustochytrid oil would have the same value and utility. There is a difference between those at 80%, 90% 95% or 98% (the examples given to Dr Wynn at [T5/568/12]);
iv) The presence or absence of a PUFA other than DHA will also affect the value and utility of an oil (Wynn XX [T6/569/10-15]). Examples were put to and agreed by Dr Wynn:
a) the presence of ARA would add to value and utility; the ARA content of the EP740 strain is very low compared to other thraustochytrids - for example strain 46A in the Barclay patent [T5/569/16 - 571/14];
b) complete absence or significant quantities of EPA would affect the value and utility of an oil from a thraustochytrid. But moderate levels would not be preferred: [T5/571/15 - 572/9];
c) presence (or more accurately, absence) of DPA may also affect value and utility; there is a significant quantity of DPA in the EP740 strain and an oil from a thraustochytrid with lower levels would have greater value and utility: [T5/572/10 - 574/4];
d) there will be variation between oils from thraustochytrids as to overall PUFA level. Those with the lowest saturated fatty acid content would be considered more valuable and useful. However, the EP740 strain has significant levels of saturated fatty acids - there will be thraustochytrids with lower levels [T5/574/ 5 - 576/18]. Dr Wynn made the point that this will particularly be so where levels of PUFAs are high - but those PUFAs may be something other than the desirable DHA.
581. In all these respects the value and utility of oil from a thraustochytrid cannot be said to be the same. I agree that the very broad range of thraustochytrids covered by the claim is plainly not enabled. EP740 does precisely nothing to help the skilled person with the task of finding other high-DHA thraustochytrids, which is a huge burden - Dr Wynn said many research teams had been trying and failing to do it for around 17 years (Wynn 1 ¶276(2)). And moreover, the range is not one that can be ignored as a range where the value and utility of all oils are unaffected by the identity of the thraustochytrid cell from which it is obtained.
582. Accordingly, the claims are not enabled across their breadth as regards their coverage of microorganisms beyond the PTA-9695 strain. They certainly come nowhere near being enabled across the entire order of thraustochytrid microorganisms.
583. Mara pointed out that the data in EP740 actually only disclose achievement of 56-57% by weight of DHA in TAG. That is the result in Tables 5 and 7 as regards Example 3.
584. Mara were prepared to take the data in Tables 15 to 21 as indicating the results that the skilled person might achieve if they undertook the mutagenesis exercise. Although routine in the sense of utilising known processes, Dr Wynn agreed that exercise is a substantial amount of work (Wynn XX [T5/559/9-14]). Having undertaken that work, DSM had data for in the region of 74 (perhaps more) ‘mutants’. Almost all produced oil with the same level of DHA in TAG (if one takes the figure for total fatty acid as indicating the level in TAG) - in the region of 56-57%.
585. The very best that was achieved was two at around 62% - mutants 73 and 74.
586. So, as Dr Wynn agreed, the data in the EP740 did not hit the top end of claims 1B or 2B - see [T5/563/21 - 565/14].
587. The evidence culminated in Dr Wynn speculating that better results would be obtained with more work. But DSM did the work on at least 74 different ‘mutants’ and did not achieve any higher level. This was also the subject of Dr Kyle’s cross examination at [T6/765/14 - 770/9]. He explained he considered the results obtained in EP740 might not be real mutants. He also explained the difficulty in this kind of work is not causing the mutation but having a mutation that is stable - see [T6/765/23-24]: “what I have found in my experience was doing that [mutagenesis] with algae, it never came back as a stable mutation”. The entire passage of cross-examination conveys the uncertainty the exercise involves. Dr Kyle accepted that the process could be used and mutants if created might produce greater increases than those seen in example 7, and that it ought to be possible to create such ‘proven mutants’. But he was never challenged on the point he made at the outset - that the problem was getting one that was stable. I remained unconvinced that stable mutants could be created which would provide the skilled person with the ability to work the invention at the top of the claim.
588. The upshot of all the evidence is that DSM failed to establish that a skilled person could, without undue effort, work the invention so as to achieve a crude oil with a DHA in TAG content at the top end of the claim, that is anywhere in the region of 63-65%, and certainly not at the top end - i.e. between 64-65%.
589. I have no doubt that the ‘limitation’ of claim 1B to ‘thraustochytrids’ is a relevant range in a Regeneron sense. The contribution of EP740 is just one thraustochytrid strain. So it is clear and I find that claim 1B is not enabled across its breadth.
590. DSM’s assertion that oils from all thraustochytrids are all of the same value or utility was just that - an assertion which was not supported by any evidence. To the extent that it was supposedly supported by evidence from Dr Wynn in Wynn 2, [74], I disagree. His [73] & [74] were the only two paragraphs in which he addressed sufficiency of EP740. In [73], Dr Wynn summarised Dr Kyle’s evidence that EP740 describes the extraction from one particular strain, ATCC PTA-9695 and that it is only this strain that is used to produce an oil with the features of claim 1B. Then Dr Wynn responded in [74] as follows:
‘However, the Skilled Microbiologist would not consider the teaching/invention of EP 740 in such a limited way, but would rather have understood that the value and utility of a microbial oil with the properties of claim 1B is the high quantities of DHA from a crude oil extracted from a Thraustochytrid biomass without further processing (i.e. they do not need to rely on expensive and complex post-extraction processing techniques). Further, the Skilled Microbiologist would recognise that these benefits would be realised irrespective of the particular Thraustochytrid strain used (provided it produces a microbial oil with the other features of claim 1B). As I note at paragraph 69 above, the value and utility of this particular feature resides in the processing benefits of using a Thraustochytrid microorganism.’
591. His point that the benefits would be realised is subject to the crucial proviso: provided it produces a microbial oil with the other features of claim 1B. That implicitly recognises that claim 1B presents the Skilled Team with a research project to find other thraustochytrid strains which provide levels of DHA of 55-65% but with <5% HDA. It also shows claim 1B is an ‘everything which works’ claim (cf Fibrogen at [73]).
592. DSM’s argument that EP740 was sufficiently enabled because they had shown one way to obtain an oil with the features specified in claim 1B misses the point entirely.
593. Finally, I should deal with DSM’s third point - see [568] above - that Mara failed to establish that there were products falling within the claim that could not be made using EP740’s teaching together with the CGK. DSM contended that it was essential for Mara to identify such an alleged product, not least so as to give DSM a chance to establish that (a) the product was not within the claim and/or (b) it was not a relevant range and/or (c) it was in fact enabled by the specification with the CGK.
594. Once again, one has to take due account of the vast breadth of the claim - all thraustochytrids. The contribution of EP740 is to provide a microbial oil with the DHA and HDA contents specified in the claim from the PTA-9695 strain. As for all the other strains of thraustochytrid which have yet to be investigated, there are two possibilities:
i) One is that there are some strains which would yield DHA and HDA content as specified in claim 1B.
ii) The other is that, in fact, there are no other strains which would yield DHA and HDA content as specified in claim 1B.
595. In either event, finding the ‘some strains’ or finding out there are no such strains would constitute a huge research project. In the circumstances of this claim, I do not believe it can be necessary to identify other specific strains which might or might not yield the high DHA levels of claim 1B.
596. At all events, in my judgment, EP740 is invalid for insufficiency.
597. EP740 was granted on a second-generation divisional application, and therefore the relevant application as filed is the “grandparent” application, namely international application PCT/US2009/001720, which I will refer to as ‘the EP740 Application’.
598. Mara’s Statement of Objections addressed all the amendments originally proposed, but I am only concerned with the proposed B amendments. The objection which remains is that claims 1B and 2B disclose a combination of features not disclosed in the application as filed.
599. The general principles were not in dispute and both sides referred to Nokia Corp v IPCom GmbH [2012] EWCA Civ 567, [2013] RPC 5 together with extracts from Gilead Sciences Inc v Nucana Plc [2023] EWHC 611 (Pat), [2023] RPC 16, Meade J.
600. DSM identified and addressed their submissions to the ‘fundamental’ question: would the skilled person learn new subject matter which was not disclosed in the application?
601. Mara went further, referring (not surprisingly) to authorities which discuss more specifically added matter by selection. I did not detect any dispute as to these principles either.
602. Whether and when selection from multiple lists can add matter was reviewed by Arnold J (as he then was) in Merck Sharp and Dohme Ltd v Shionogi and Co Ltd [2016] EWHC 2989 (Pat) at [288]–[293], where he said:
‘[291] … Where a specification contains a series of lists of variables, but does not point to a particular combination of choices from the respective lists, an amendment that narrows to that particular combination will ordinarily add matter. As counsel for MSD submitted, this principle is traceable back to the important early decision in T 12/81 Bayer/ Diastereomers [1979-85] EPOR B308. In that case, the Board was considering the novelty of a selection from two lists. It held at [13]:
“However, the disclosure by description in a cited document of the starting substance as well as the reaction process is always prejudicial to novelty because those data unalterably establish the end product. If on the other hand two classes of starting substances are required to prepare the end products and examples of individual entities in each class are given in two lists of some length, then a substance resulting from the reaction of a specific pair from the two lists can nevertheless be regarded for patent purposes as a selection and hence as new.”
Such a selection from two lists can be novel for the purposes of patentability, and by the same logic it will also constitute added matter if it was not disclosed in the application as filed.’
603. The issue of the extent of narrowing, and whether it is permissible or adds matter, was further considered by Meade J in Gilead Sciences Inc v Nucana Plc [2023] EWHC 611 (Pat), [2023] RPC 16 at [236]-[278]. At [253] he identified the following principles:
i) Do the deletions single out a particular combination of specific meanings, i.e. a hitherto not specifically mentioned individual compound or group of compounds?
ii) Or, do the deletions merely maintain the subject matter as a generic group of compounds differing only from the original group by its smaller size?
iii) It is relevant to consider whether the deletions 'generate another invention'. Another invention will be generated if the smaller group provides a technical contribution.
604. In that case Meade J. was concerned with the narrowing of a Markush formula, so he framed the principle in terms of compounds, but the same points apply to any narrowing amendment.
605. Meade J also drew attention to a point from Idenix v Gilead [2014] EWHC 3916 (Pat), in which Arnold J attributed importance to whether the narrower class was one that had undisclosed characteristics as regards validity:
‘247. A similar issue was considered by Arnold J in Idenix v Gilead. At [609]–[610] Arnold J found in relation to some proposed amended claims that deleting options from the possibilities for R1 and R2 (it does not matter what they were) disclosed a new sub-class of compounds, not previously disclosed. He also accepted a submission that this was made worse by the fact that it was done to remove from the granted claims compounds which, the patentee's own expert had said, were not plausibly effective.
248. The Court of Appeal said ([2016] EWCA Civ 1089 at [206]–[210]) that Arnold J was right for the reasons he had given.’
606. Meade J returned to this point in rejecting a contention that G2/10 had shown that whether there is a 'different invention' was not part of the law of added matter:
‘274. I do not see anything inconsistent in G2/10 with the notion that when asking whether an amendment adds matter, which is the fundamental question, it will be relevant to ask whether it presents a different invention, and that part of that inquiry may be whether it provides a new technical contribution. One is not inquiring whether there is a new technical contribution instead of asking whether there is added matter, but simply recognising it as a likely symptom of there being added matter.
275. Furthermore, there is no sign in the EPO's case law of its thinking that G2/10 meant that the existing law about deleting from lists was wrong. The Case Law book cites decisions from before and after G2/10 and they are all to the same effect.
276. I also noted above that in Idenix v Gilead Arnold J accepted an argument that the provision of a technical contribution across the scope of the claim for the first time was relevant to added matter, and the Court of Appeal upheld him.’
607. Finally, Mara drew attention to the fact that Meade J has recently considered selections from lists in Modernatx, Inc.v Pfizer Limited [2024] EWHC 1695 (Pat) at [120]-[146]. At [122], he referred to the ‘gold standard’ as set out in the Case Law of the Boards of Appeal of the EPO (10th Ed) at 1.3.1, and at [126] he said this:
‘126. The gold standard formulation above refers to the whole document and to the common general knowledge. I accept, of course, that the whole document has to be considered, but that does not mean that it is a reservoir from any part of which a feature can be taken to combine with a feature from some other part, in the absence of a clear teaching to do so. Similarly, the CGK informs, as ever, what the skilled person understands from the document but it does not make the CGK a reservoir from which features can freely be drawn to be plugged in at will. …’
608. Slightly later, Meade J addressed ‘Selection from multiple lists’. He reminded himself the approach is not mechanistic, the underlying question remains whether there is new information disclosed and the EPO caselaw recognises that there may be implicit or explicit ‘pointers’ to a combination. In this regard, at [139] Meade J. said:
‘139. I do not think there is any conceptual limit on what may be a pointer in this sense, but a particularly common one is a statement of preference within a list in the document in question. I note however that in a number of the EPO cases it was held that there was added matter in combining a preferred member of one list with a member of another list for which no preference was expressed. In general, too, what the EPO looks for is a pointer to the combination; this cannot be an absolute rule, but it makes sense. See for example the references to T2273/10 and T1032/12 on pages 524 and 525 of the Case Law.
140. Other pointers could potentially be dependent claims (see e.g. T583/93), or members of a list which feature strongly in the preferred embodiments (in T583/93 the Board linked these by saying that dependent claims are inherently indicators of preferred embodiments - see 4.7 of the reasons), but again there cannot be a rigid rule. …’
609. He went on to consider the role of the CGK and he accepted that it was not legitimate to say that there was a relevant pointer merely because the CGK would be that one among a number of choices was desirable. He pointed out that:
‘142…. This is the difference between using the CGK to assess the skilled person's understanding of what is disclosed, and using the CGK as a reservoir of additional disclosure. The former is mandatory and the latter is illegitimate.’
610. Finally, he pointed out that ‘pointers’ are a facet of the gold standard and not a replacement of it.
611. It seems to me that all the points made by Meade J. to which my attention was drawn are entirely valid and I propose to follow them.
Application to the facts
612. As will be seen, the arguments on added matter morphed and developed as the trial progressed. I start with DSM’s case as to why there was no added matter as pleaded which, naturally enough, Mara addressed in its Opening Skeleton. In closing, DSM took a different tack which Mara had to address in their oral closing.
613. In response to Mara’s objection, DSM’s pleaded case was somewhat complicated, but it pleaded reliance on the following support for claim 1B:
i) claims 23 and 26, paragraphs [0022], [0024], line 4 of paragraph [0054] which defines a triglyceride fraction comprising at least 55 wt.% DHA;
ii) also in [0054] the passage on page 16 line 2, which defines a triglyceride fraction comprising from at least 55 wt.% to 65 wt.% DHA;
iii) Example 3, Tables 6 and 8 of the EP 740 Application;
iv) in addition to the entire EP 740 Application, including paragraphs [0001], the first sentence of [0035] and paragraph [0051] which recite that the invention is directed to microbial oils of thraustochytrid microorganisms’ and;
v) paragraph [0052] of the EP 740 Application.
614. I can accept the general thraustochytrid point in iv) above.
615. Next, it is convenient to address the reliance on Example 3, Tables 6 and 8.
616. Mara submitted that these Tables cannot possibly provide basis for the DHA % feature. DSM’s EP740 opening skeleton suggested at ¶36(d) that the skilled person could tell from Example 3 that 55 to 65% is an important range because the figures in Examples 3 were 58.1% and 60.4%.
617. Mara had two points in response, both of which I accept:
i) First, even if the quoted figures were correct, they cannot provide basis for the range in what was claim 1A (whether alone or in combination with [0054]).
ii) Second, in any event, the figures relied upon by DSM are not correct - on the claimed measure, the correct figures are 56-57% (as explained in [382] above).
618. That leaves, in essence, the identified passages in [0054], plus [0052].
619. [0054] of the EP740 Application [A2/4/204] starts as follows (emphasis added to text understood to be relied on, and carriage return added for clarity between the first two sentences):
‘[0054] In some embodiments, the microbial oil and/or one or more fractions thereof, selected from the triglyceride fraction, the free fatty acid fraction, the sterol fraction, the diglyceride fraction, and combinations thereof, comprises at least about 40%, at least about 45%, at least about 50%, at least about 55%, at least about 60%, at least about 65%, at least about 70%, at least about 75%, or at least about 80% by weight DHA.
In some embodiments, the microbial oil and/or one or more fractions thereof selected from the triglyceride fraction, the free fatty acid fraction, the sterol fraction, the diglyceride fraction, and combinations thereof, comprises from about 40% to about 45%, about 40% to about 50%, about 40% to about 60%, about 50% to about 60%, about 55% to about 60%, about 40% to about 65%, about 50% to about 65%, about 55% to about 65%, about 40% to about 70%, about 40% to about 80%, about 50% to about 80%, about 55% to about 80%, about 60% to about 80%, or about 70% to about 80% by weight DHA.’
620. At this point, it is convenient to move to the way DSM put their case in their closing argument.
621. DSM acknowledged that the EP740 Application discloses a number of different microbial oils but contended that an oil with the particular features of the amended claims is clearly and unambiguously disclosed. DSM contended that the skilled person learns nothing new from the amended claim that they would not have readily understood from the Application.
622. The starting point for DSM was [0022] and claim 23 of the Application, which both disclose:
‘a microbial oil comprising a triglyceride fraction of at least about 70% by weight, wherein the docosahexaenoic acid content of the triglyceride fraction is at least about 60% by weight’
623. DSM sought to tackle the differences between that disclosure and Claim 1B of EP740 in the following submissions.
624. First, DSM contended that claim 1B makes express that the microbial oil is a “crude oil ... without further processing” extracted from a “Thraustochytrid microorganism”. This is not new information, for that is the focus of the Application. The Application is directed specifically to Thraustochytrid organisms and crude oil derived from them: this is clear from the title of the Application, [0001] and the “need” which the Application addresses ([0006]). The section on microbial oils (starting at [0051]) begins by saying that the oil can be extracted from a Thraustochytrid biomass (i.e. a crude oil). There are a few isolated references to the crude oil being further processed: [0052], [0057], [0059] and Example 5, but these simply reinforce that the focus of the Application as a whole is on the fatty acid composition of Thraustochytrid crude oil. Example 5, for instance, simply applies standard RBD and dilution to the crude oil.
625. Second, the DHA in TAG is specified as 55% to 65%. This is not new information. That range is clearly and unambiguously disclosed in the general teaching at [0054]. Moreover, the skilled person looking at claim 23 and its focus on the % DHA in TAG would understand from Example 3 that the range 55-65% DHA in TAG is important as it is the narrowest disclosed range that encompasses both crude oil samples in Example 3. Example 4 specifically analyses the TAG fractions of the samples in Examples 3, further confirming that it is the TAG fraction that is significant.
626. Finally, the oil has less than 5% heptadecanoic acid (“HDA”). Dr Kyle explained that it would be considered surprising that this feature is mentioned as this would be the expectation for virtually all high PUFA microbial oils. The skilled person therefore understands that this is a feature of the microbial oil of the invention. In addition, Example 7 measures the HDA content of ATCC PTA-9695 (and mutants thereof), all of which have well below 5% HDA, and as Dr Kyle said, this suggests that samples A1 and A2 in Example 3 would also have had less than 5% HDA. Consistent with all of this, [0024] and claim 26 disclose this feature in combination with the microbial oil of [0022] and claim 23. (Thus, in a sense, one could start with claim 26 and eliminate the debate over HDA altogether.)
627. DSM contended that the above analysis is the answer to Mara’s point that 55% to 65% DHA in TAG is disclosed in [0054] in the context of a long list of other possible claim features (e.g. in some embodiments the EPA content is limited to 10% or less). But claim 23 already discloses an oil which is characterised by its % TAG, its % DHA in TAG and no other features (and claim 26 discloses an oil which is characterised by its % TAG, its % DHA in TAG, <5% HDA and no other features).
628. As to the rest of Mara’s points set out in their Opening Skeleton, DSM submitted as follows:
i) Mara suggested that “from at least 55% to 65%” DHA in TAG “is not disclosed at all” in the EP740 application. DSM’s response was that this was a pedantic point amounting to little more than saying the specific words differ. [0054] of the Application discloses a DHA in TAG range of “from ... about 55% to about 65%”. DSM contended there is nothing new taught by the words “from at least”, nor by the conventional avoidance of the word “about”. Both describe a range of 55% to 65% (to the nearest whole percent). DSM said that no-one had suggested any other meaning.
ii) DSM repeated their argument that the amendment to 55% to 65% DHA in TAG does not single out a group of oils not specifically mentioned in the Application. On the contrary, DSM submitted that range captures the microbial oils at the heart of the disclosure.
iii) Mara appeared to rely on DSM’s alleged intention for making the amendment (Mara’s Opening Skeleton ¶247). However, DSM’s motivation for making an amendment is irrelevant to its permissibility.
iv) In any event, DSM submitted, asking whether there is another invention, or technical contribution, does not replace the test for added matter (see Gilead [274], cited above). This was the main point made in Mara’s Opening Skeleton ¶247: that restricting to the smaller group did generate another invention. Mara said this was the point of the amendments - to identify a group of oils that is now said to provide a technical contribution across the scope of the claim. Mara gave two examples from Dr Wynn’s evidence:
a) First that Dr Wynn explained that the long-available DHASCO contained up to about 50% DHA (which was almost all triglyceride), and he contrasted this with the >55% DHA in triglycerides disclosed in EP740 for its strain (Wynn 1 ¶250(2)).
b) Second, Dr Wynn also acknowledged a CGK Thraustochytrium strain that contained 52% DHA (see the table in [104] above). The new claimed lower limit of 55% is therefore relied on to provide a technical contribution across the scope of the claim.
On this basis, Mara submitted that the amendments to claim 1B represent an illegitimate narrowing of the claim that adds matter.
v) DSM’s response was that it is important not to lose sight of the fundamental question and, furthermore, that it is difficult to understand why Mara considers that their two examples indicate that the amended claims contain added matter: (i) DHASCO is a Crypthecodinium cohnii oil and therefore outside the teaching of the Application which relates to Thraustochytrids; and (ii) the Thraustochytrium strain with 52% DHA of total fatty acids but only 15% total fatty acids evidently does not contain a high TAG fraction, nor is the 52% a measure of DHA in TAG (Wynn 1 ¶89; Kyle XX T6/756/18 - 759/4).
629. Finally, DSM submitted that Claim 2B is simply a narrowing of the % DHA in TAG range disclosed in claim 23 of the Application to add an upper limit of 65%. This introduces no new information.
630. In Mara’s oral closing, Dr James Whyte made the submissions on this part of the case (and on the amendments). He addressed directly DSM’s submissions which I summarised in [624]-[629] above.
631. He pointed out, correctly, that claim 23 is to a microbial oil which is defined in [0052] (which is the same as [0029] in EP740, which I set out in [376] above). That paragraph presents a number of options, out of which DSM select the ‘crude oil …without further processing’. His point was that the reader of the application would not conclude that it is all about crude oils.
632. His second point concerns the selection of thraustochytrid limitation. This is not mentioned in claim 23, in contrast to other claims where thraustochytrids are explicitly mentioned - claim 36 claims a method for producing a microbial oil … comprising (a) growing the isolated thraustochytrid microorganism of any one of claims 1 to 9…, where those claims are limited to one of the PTA strains mentioned in the specification - 9695, 9696, 9697 and 9698.
633. Third, the DHA in TAG fraction. Dr Whyte drew attention to claims 20-23, each of which claim different requirements for the content of the TAG fraction. His point here was that is a set of four claims with four specific, clearly and unambiguously disclosed requirements for the TAG fraction, but DSM does not want any of them, instead ‘going on a hunting expedition in the specification’. Dr Whyte pointed to pages 14-19 of the Application containing long lists of different parameters with different % or ratios in countless combinations, with no preference for any particular value or combination expressed. He invited the conclusion that the only combinations which are singled out and individualised in the Application are those in claims 20-23. Again, his point is that DSM happily discards the DHA range set out in claim 23 and substitutes for it some other disclosure which you can only find if you know what you are looking for.
634. So Dr Whyte says that DSM have to go on a hunting expedition: first, for the TAG fraction at all, which they have to pick out from the variety of fractions disclosed in [0053], and from the TAG fraction, they have to pick out ‘at least about 70% by weight’. Then DSM have to turn to [0054] and select the TAG fraction from the others mentioned and then select the levels they want - ‘about 55% to about 65%’ DHA by weight. This however involves a selection of DHA from the other PUFAs mentioned in [0054] i.e. selecting no limitation regarding (a) EPA content, (b) DHA to EPA ratio, (c) ARA content, (d) DHA to ARA ratio, (e) DPA n-6 content, (f) DHA to DPA n-6 ratio etc, regarding linoleic, linolenic, eicosenoic and erucic acids.
635. Dr Whyte counted 6 selections to get to the DHA range and a further 3 to get to the HDA level of ‘about 5% or less of’ HDA by weight, but he pointed out that one still has not got to the language now featured in claim 1B. He contrasted the language in the application with the language in claim 1B:
i) ‘about 55% to about 65%’ vs ‘from at least 55% to 65%’
ii) ‘about 5% or less’ for HDA vs ‘5% by weight or less of’ HDA.
636. In this regard, he cited the judgment of Arnold J. in Napp Pharmaceutical v Dr Reddy’s Laboratories [2016] EWHC 1517 (Pat), [2017] RPC 4 at [125], upheld on appeal: [2016] EWCA Civ 1053, [2017] RPC 5 at [75], for the proposition that it would be contrary to principle to treat the word ‘about’ as if it was not there because, on the ordinary principles of construction, it must mean something. i.e. a small degree of permitted variation from the stated value compared with the situation if the word was not there. Whatever small degree of fuzziness is imported by the word ‘about’, he submitted the words ‘at least’ have a different flavour and meaning. Dr Whyte pointed out that Ms Nezami relied on the fact that the ‘at least’ wording for the DHA range is narrower than the ‘about’ wording, but, as he also pointed out, that means the disclosures are different.
637. Dr Whyte concluded that each of the differences he pointed out between claim 23 and claim 1B are not clearly and unambiguously disclosed in the Application, so there is added matter in claim 1B.
638. Dr Whyte submitted the situation is even worse for claim 2B. First, because DSM did not even attempt to identify a basis for it. Second, in DSM’s closing, they said that claim 2B is simply a narrowing of the %DHA in claim 1B that had an upper limit of 65%, without identifying any basis for it.
639. Dr Whyte then turned his attention to DSM’s reliance on Example 3 to justify the range of 55-65% in claim 1B, DSM’s argument being that it is the narrowest disclosed range that encompasses both of the crude oil samples in Example 3. However, as Dr Whyte pointed out, these data points are 56/57% in the two experiments, using the correct measure. So, even if this were an acceptable approach, the narrowest range covering the two data points in [0054] is ‘about 55% to about 60%’.
640. Dr Whyte’s next point was that there is nothing in Example 3 to tell the skilled reader that it is the TAG fraction they are supposed to be interested in. The tables list all the possibilities mentioned in [0054], with no pointer to it being the TAG fraction that should be focussed on. The same point applies to the focus on DHA.
641. Next, Dr Whyte dealt with a suggestion from DSM that there was some support in Example 7 for the HDA integer. Table 15 gives an HDA % of 0.12 in PTA-9695. But amongst the options listed in [0054] for HDA, there is 1% or less. So Dr Whyte submitted that if it was legitimate to infer anything from Example 7, it would point to ‘less than 1% HDA’ and not to ‘less than 5%’. Furthermore, there was no support for ‘5% or less’ from the CGK because the CGK was that there was no or negligible HDA which does not get one to ‘5% or less’ even if it was legitimate to use the CGK as a reservoir (see [609] above).
642. Mara’s next point relied on Meade J.’s indication that if the amendment results in a new invention/technical contribution, that is a likely symptom of there being added matter. In this case, Mara hardly need this point.
643. In relation to Fabritius, DSM now say that their contribution is the new group of crude oils in claim 1B. This, Dr Whyte submitted, was to claim a class of oils that is said to provide a technical benefit when previously the Patent did not do so - a symptom of added matter.
Conclusion on Added Matter
644. Ultimately, the conclusion that both claims 1B and 2B add matter is, in my view, inescapable for all the reasons explained by Dr Whyte and summarised above.
EP801
645. By way of a reminder, EP801 is entitled “Extraction of lipid from cells and products therefrom” with an earliest priority date of 01 June 2010 which is not challenged. It is said to relate to a microbial oil extraction process which solves the emulsion problem without the use of organic solvents. Indeed, DSM’s case throughout was founded on the proposition that EP801 claimed a solventless extraction process. This resolves to one of the key issues of construction of claim 1A.
646. This short section sets out agreed areas of CGK which applied at the EP 801 Priority Date, namely 1 June 2010, in addition to the matters set out above.
647. Between 2002 and 2010 there was an increasing interest in moving away from the use of hexane, in particular due to factory safety and consumer marketing.
648. Certain companies, including Martek, started to use isohexane as an alternative. Isohexane is still a volatile and flammable organic compound, but was not classified (at least by the United States regulator) as a hazardous air pollutant. Isohexane was therefore a safer alternative, but there was still a desire to move to even less harmful alternatives. There was also increasing interest in the FRIOLEX process (explained above) between 2002 and 2010.
Solventless techniques / emulsions (EP 801 Priority Date - 1 June 2010)
649. The issue was whether the reason there was no commercial aqueous extraction method (without any solvent) in 2010 was because (i) there was no known effective way of breaking the emulsion, or (ii) commercial reasons.
650. In this formulation of the issue, it should be noted it is concerned only with whether a solventless extraction method had been developed on a commercial scale.
651. DSM relied on the evidence of Mr Dueppen which was to this effect:
‘Overall, there was no recognized effective way of breaking the
emulsion that would form in an aqueous extraction process on a commercial scale. As such even by 2010 the Skilled Bioprocessing Engineer would be aware that solventless extraction was not being used in practice, mainly due to this emulsion problem.’ Dueppen 1, [76].
652. Accordingly, DSM submitted that the industry was looking for an entirely aqueous method, but at the priority date there was still no known effective way of breaking the emulsion without an organic solvent.
653. DSM also submitted that Dr Kyle did not attempt to defend his claim in Kyle 2 ¶14 that there “were numerous techniques available for breaking an emulsion”, at least so far as concerns the priority date of EP801. As I mentioned earlier, I am not inclined to rely on any of Dr Kyle’s evidence unless corroborated.
654. In this regard, Mara did not rely on Dr Kyle’s evidence. Instead, they relied on evidence given by Mr Dueppen. Mara contended as follows.
655. Emulsions did not form when using hexane extraction, so emulsions were not a concern when using hexane. Emulsions could form in an aqueous environment, and particularly so with mechanical methods such as homogenisation that caused high shear, which also generated heat that had to be dealt with (Dueppen XX [T2/117/20 - 120/8]).
656. Following the evidence, it is clear that the following were CGK methods for seeking to break an emulsion:
i) Use of a polar organic solvent such as isopropanol (as in the FRIOLEX process). This worked by the water-miscible solvent making the aqueous phase even more polar, and so less favoured by the non-polar lipids such as TAGs (Statement of Agreed CGK ¶71; Dueppen XX [T2/96/7 - 97/3]).
ii) Addition of salt. The mechanism of this was to increase the density of the heavy (water-containing) phase and encourage better separation, particularly upon centrifugation (Statement of Agreed CGK ¶71; Dueppen XX [T2/95/12 - 96/6]).
iii) Heating, which worked by increasing the energy in the system and increasing the rate at which droplets of oil can meet and coalesce (Dueppen XX [T2/97/4-18]).
iv) Stirring (gentle agitation), which works on a similar principle to hearing, by increasing the rate at which droplets may come into contact and coalesce (Dueppen XX [T2/97/11 - 98/5, T3/340/10-12]). Vigorous agitation, however, can promote formation of emulsion (Dueppen XX [T2/92/13/-20, 98/6-18]).
v) Centrifugation, which when used alone could break weak emulsions, and for stronger emulsions would be used along with other approaches (Dueppen 2 ¶37).
vi) Combinations of the above. For instance, heating and stirring would have been used with one of more of the other techniques. Also, centrifugation amplifies the effect of gravity, so works in combination with the increased density differential caused by addition of salt.
657. Mara observed that Mr Dueppen seemed to become a bit confused during his cross-examination about whether an emulsion comprises a single phase or two phases. He suggested that in a strong emulsion there was no density difference between the two elements of the emulsion because the emulsion could not be broken by centrifugation, and that the two components therefore formed a single phase (Dueppen XX and question from the Court [T2/193/18 - 197/6]). While this may not matter to the issues in the case, Mara submitted this is wrong, contending that it was contrary to the agreed CGK, and contrary to Mr Dueppen’s own evidence as to what an emulsion is. An emulsion is a mixture of two (or more) liquids, where one liquid is present as microscopic droplets distributed throughout the other (Statement of Agreed CGK ¶74 [B1/35/302]). There is inevitably still a density difference between the two elements of the emulsion (i.e. the droplets and the liquid in which they are distributed). Mr Dueppen’s written evidence correctly agreed with EP801 that an emulsion is a mixture of two or more immiscible phases or layers, dispersed within each other (Dueppen 1 ¶185(b)), and hence is not a single phase.
658. As to the question of why there was no commercial aqueous extraction method (without any solvent) in 2010, Mr Dueppen readily agreed in cross-examination with the existence of commercial barriers, namely Martek’s patent position and its commercial dominance (Dueppen XX [T2/204/4-24], by reference to Kyle 2 [14]).
659. In closing submissions, DSM acknowledged those factors but contended that they did not explain why Martek or anyone else in the industry, or academia, did not develop an aqueous method before 2010. DSM’s final point was that in any event, those reasons relate only to DHA oils and not other PUFA rich oils. Those points were not explored in any evidence, so I leave them out of account.
660. In my judgment, the evidence established the following:
i) The various techniques covered in [656] above were CGK as possible methods for breaking an emulsion. Which ones would actually work effectively in practice would depend on the strength of the emulsion. If in doubt, the Skilled Team would conduct a simple lab test at the bench to see which techniques or combinations would work effectively.
ii) As a matter of fact, no solventless method had been developed at a commercial scale.
iii) But the absence of such a method at a commercial scale was adequately explained by the commercial barriers, namely Martek’s patent portfolio.
The extent to which centrifugation alone (i.e. in the absence of additional steps) can be expected to break the emulsions formed when cells are lysed in aqueous conditions in a microbial oil production process. [Kyle 1 ¶122; Dueppen 2 ¶37; Kyle 2 ¶20].
661. Following cross-examination, there was not really much of a dispute left on this point. It all depends on the context.
662. So far as weak emulsions were concerned, Dr Kyle said that centrifugation alone could break a weak emulsion - Dr Kyle gave as an example an emulsion of olive oil and water. Mr Dueppen accepted that example. His point was that in microbial oil production the emulsions were simply too strong to break by centrifugation alone. I understood his point to relate to commercial processes in which brute force mechanical methods of lysis were used e.g. homogenisation.
663. Mr Dueppen also said that, for stronger emulsions, the presence of emulsifiers stabilizes the oil-water interaction, and prevents centrifuging separating based on the density differential between oil and water. This is why he said, for example, in the FRIOLEX process isopropanol is used, as an isopropanol/water mixture is more polar than water alone, and that helps weaken the interaction with non-polar oil, allowing it to be separated with the aid of centrifugation.
664. Overall, Mr Dueppen maintained his scepticism in relation to claims that centrifugation alone would break an emulsion in a microbial oil production process.
665. In view of my acceptance of the range of techniques set out in [656] above, this point is largely academic, and, as I said above, much depends on the context, in particular the strength of the emulsion.
pH values
666. Due to the issue of interpretation which I outline below, Mara contended that the Skilled Person would be familiar with pH being a logarithmic scale, meaning that for each whole unit or integer of pH, there is a ten-fold change in concentration of the hydrogen ions. Accordingly, Mara submitted that it is far from the case that a pH of 7.5 differs from a pH of 8 by a factor of 0.5 / 8, or about 6%. Instead, a pH of 7.5 is over threefold more acidic than a pH of 8. Even a 0.1 pH unit change corresponds to a change in hydrogen ion concentration of 26%.
667. It cannot be disputed that pH has a logarithmic scale, but it remains to be seen the significance of this for the Skilled Person in this case.
668. Two further CGK issues concerning pH values emerged in the course of closing argument. The first issue was whether the Skilled Team, when operating a process in this field in which pH mattered (e.g. because a process step required the mixture to be at or above a particular pH), they would operate to the nearest whole pH value.
669. DSM in closing asserted this to be the case, but did not identify any passage in any of the expert evidence to back this up.
670. The second issue concerns how the pH of a lysed cell mixture would vary with time. In my view, the Skilled Team, but in particular the Skilled Bioprocessing Engineer would be well aware that the pH of a composition of microbial cells is likely to change as lysis of the cells continues and would be alive to the need to add further chemicals in order to sustain a desired pH value or range.
671. The claims in issue, with the proposed amendment shown underlined (and claim 5A included in square brackets because of the dependency in claim 6A), are:
1A. A process for obtaining a lipid from a microbial cell, said process comprising:
a) lysing a cell to form a lysed cell composition;
b) adding a base to the lysed cell composition, raising the pH of the lysed cell composition to 8 or above to demulsify the cell composition;
c) one or more of c1, c2, c3 and c4;
c1) adding a salt to the lysed cell composition to demulsify the cell composition
c2) heating the lysed cell composition to demulsify the cell composition
c3) agitating the lysed cell composition to demulsify the cell composition
c4) adding a second base to the lysed cell composition to demulsify the cell composition;
and
d) separating a lipid from the demulsified cell composition;
wherein the lipid contains less than 5% by weight of an organic solvent and wherein the lysing comprises enzymatic treatment.
[5A. The process according to any preceding claim, wherein the process comprises heating the lysed cell composition to demulsify the cell composition.]
6A. The process according to claim 5A, wherein the heating is performed after the adding a salt.
7A. The process according to any preceding claim, wherein the process comprises agitating the lysed cell composition to demulsify the cell composition.
672. Although DSM addressed a whole series of construction issues in their opening, by the time of closing argument, the live issues on construction had reduced to the following:
i) First, the issues which fell under the term ‘the order of the steps’ which included the interpretation of ‘lysed cell composition’ and ‘raising’.
ii) Second, the pH limit. Although the argument on the pH value focussed on the expression ‘8 or above’ those words need to be considered in the context of the claim as a whole but also the phrase in which it appears, namely: raising the pH of the lysed cell composition to 8 or above to demulsify the cell composition.
iii) Third, the 5% limit on the weight of organic solvent which is a point of significance in view of DSM’s approach to the claim.
673. As is usual, some of these issues relate to prior art attacks and some to infringement.
674. So far as validity of EP801 is concerned, in closing, Mara dropped its insufficiency attacks, saying they had served their purpose. Mara maintained the following attacks on the validity of EP801:
i) Alleged anticipation by Kobzeff, alternatively obviousness.
ii) Alleged obviousness over Hendrik.
675. As I describe below, the infringement issues narrowed in the lead up to trial and during DSM’s Opening, but the interplay between infringement and validity continued to develop. Both sides outlined the infringement issues on EP801 in their written openings. However, in correspondence on 25 September 2024, Mara conceded infringement of EP801 by some processes, leaving the only live issue on infringement concerned with the construction of ‘pH… 8 or above’. The concessions were carefully worded, with Mara accepting that process 1 fell within the scope of protection of claim 1A and the DNI on process 10 was no longer pursued for the same reason. These concessions were on the basis that DSM would not resile from their ‘broad construction of claim 1A and as supported by Mr Dueppen’s evidence’.
676. However, the issues which had been or were live on infringement had consequences as to the breadth of the claim:
i) It was DSM’s case that a pH of 7.5 (or above) would suffice for infringement, on a normal construction of the claim.
ii) Equally, it was DSM’s case that step (b) was satisfied simply by the pH being at the required level, there being no need for ‘raising’, on infringement by equivalence.
677. This second point was clearly explained in Mr Dueppen’s evidence on DSM’s case of infringement by equivalence. He noted that for one process (Process 13) there were potentially batches in which the pH raise had occurred before the addition of the enzyme. In that context, his evidence (quoted in DSM’s Opening Skeleton) was that:
‘…it is not the act of changing the pH in EP 801 which aids demulsification and recovery of an oil that is not dispersed in an emulsion. Rather it is the state of being at that elevated pH, as pH is simply a physicochemical property and measure of the acidity/basicity of a solution. If the pH is already at 8 or above when a lysed cell composition forms the emulsion which arises may be weaker, raising the pH to 8 or above would weaken an existing emulsion by the same action.
289. Accordingly, maintaining the pH of a lysed cell composition that is already at pH 8 or above has, from a technical perspective, the same effect as raising the pH of a lysed cell composition to that same level.’
678. DSM also relied on this equivalence case as a fallback on Process 1, although their primary position was that that process infringed on a normal construction.
679. Mara also originally claimed a Declaration of Non-Infringement in relation to Process 10 in which the pH is first raised to the target pH and only then is the enzyme added.
680. For their part, Mara’s Opening argued that DSM’s broad construction of their claim made EP801 invalid over the prior art - Kobzeff and Hendrik. Kobzeff was said to anticipate on the basis that his disclosure of a pH range of 5-9 covers a pH of 5 and 9 and all values in between.
681. There was a passing reference in DSM’s Opening Skeleton to the effect that there was no Formstein defence because any obvious implementation of Kobzeff would involve isopropanol and therefore be outside EP801 - this contention obviously being dependent on the construction of the final integer of the claim as granted.
682. These issues exploded into life in about the last hour of the trial, as closing submissions on EP801 were drawing to a close. DSM complained that Mara were trying to run a Formstein defence, but Mara submitted it was more a Gillette defence because they had accepted DSM’s contention of infringement where the pH was raised to 8 even before lysis starts.
683. Mara’s argument was founded on the basic principle that it is generally accepted that a valid patent cannot prevent a defendant from doing an act that is obvious at its priority date. Mr Speck KC identified three possible positions which DSM might have been taking:
i) The first he described as a radical submission that DSM can have a claim that remains valid because what is obvious is not within it on a normal construction but can be infringed by the doctrine of equivalents by a variant which was obvious.
ii) Alternatively, he said that DSM’s position might be that whenever a situation arises where it can be seen that the application of the doctrine of equivalents covers an obvious product, the doctrine is to be disapplied to save the patent in every case.
iii) The third possibility is raised by the particular circumstances of this case where Mara, having seen how DSM’s infringement allegations were framed and supported, conceded infringement. DSM bank the concession and then change their position on the scope of protection of the claim.
684. For DSM, Mr Abrahams KC submitted in his reply speech that if the point arises, the solution lies in the Formstein defence, with the result that the patent remains valid. He referred me to the analysis of Birss LJ in Facebook v Voxer [2021] EWHC 1377 (Pat) at [209]-[217], esp. [216]. He pointed out that it was far too late for Mara to seek to plead and rely on a Formstein defence.
685. I permitted Mr Speck KC to respond to the new points which had been raised. He pointed out that Birss LJ’s dictum was obiter and he had not actually decided the point. He submitted that the solution that the patent was always valid could not be right. He invited me to assess the merits of the arguments.
686. Following the conclusion of the trial, each side made further short written submissions, also referring me to the discussion by HHJ Hacon in Technetix v Teleste [2019] EWHC 126 (IPEC) at [84]-[100] and [126]-[133]. I have also had regard to Celltrion v Genentech [2025] EWHC 174 (Pat), another decision of HHJ Hacon (this time sitting as a Judge of the Patents Court), in which he discussed an alternative argument put forward by Celltrion of anticipation by an equivalent of the invention at [74]-[108], where he concluded that the argument had no basis in English law.
687. I will see where my decisions on this part of the case lead, but at the outset I am inclined to assess the merits of the arguments if it is necessary to do so, not least for the reason explained by Floyd LJ in Fujifilm at [56], when he was discussing the Gillette defence:
‘It is, we would accept, still not the practice to adopt Lord Moulton's approach to deciding conventional patent actions where both validity and infringement are in issue. The court will resolve those issues individually by reference to the claims of the patent, rather than take the short cut of deciding whether the defendant's product is old or obvious. That is because, as we think Lord Moulton was recognising, the validity of a granted patent involves more than just the private interests of the parties. If the patent is indeed to be impaled on the validity horn of Lord Moulton's dilemma, then it is in the public interest that it be decided and the patent revoked. That same policy is visible in Traction Corporation v Bennett (cited above). That consideration does not, however, detract from the potential usefulness of the principle that Lord Moulton was espousing. In a conventional patent action a determination that there is nothing new or inventive about the defendant's product may operate as a cross-check on the outcome of the action as a whole.’
688. The normal rules of construction of patent claims apply. I have them well in mind and do not propose to cite the relevant well-known authorities.
689. As is well-known, the normal principles of construction apply to numerical limits, but it is nonetheless useful to remind myself of Floyd LJ’s review of the principles in Jushi Group Co., Ltd v OCV Intellectual Capital, LLC [2018] EWCA Civ 1416, [2019] RPC 1 at [36]-[39]. He cited with approval the decision of Kitchin LJ in Smith & Nephew Plc v ConvaTec Technologies Inc [2015] EWCA Civ 607, [2015] RPC 32 at [38].
690. DSM relied directly on the review by Kitchin LJ in Smith & Nephew v ConvaTec [2015] EWCA Civ 607, [2015] RPC 32, at [16]-[38] and in particular his concluding paragraph:
‘38. As I have said, the approach to be adopted to the interpretation of claims containing a numerical range is no different from that to be adopted in relation to any other claim. But certain points of particular relevance to claims of this kind do emerge from the authorities to which I have referred and which are worth emphasising. First, the scope of any such claim must be exactly the same whether one is considering infringement or validity. Secondly, there can be no justification for using rounding or any other kind of approximation to change the disclosure of the prior art or to modify the alleged infringement. Thirdly, the meaning and scope of a numerical range in a patent claim must be ascertained in light of the common general knowledge and in the context of the specification as a whole. Fourthly, it may be the case that, in light of the common general knowledge and the teaching of the specification, the skilled person would understand that the patentee has chosen to express the numerals in the claim to a particular but limited degree of precision and so intends the claim to include all values which fall within the claimed range when stated with the same degree of precision. Fifthly, whether that is so or not will depend upon all the circumstances including the number of decimal places or significant figures to which the numerals in the claim appear to have been expressed.’
691. DSM also referred to the outcome in the authorities the subject of that review, as well as the outcome in Smith & Nephew itself, where the number of decimal places featured in the claim was influential. DSM also mentioned my own decision in Sandoz v Biogen [2024] EWHC 2567 (Pat), where, at [301]-[307] I interpreted ‘>1.5’ in the claim as a bookend, it being highly relevant that the specification included a figure which made clear that values between 1.45 and 1.5 were intended to fall outside the claim.
692. DSM submitted I should apply ‘the general rule’: i.e. because claim 1A specifies the pH to a whole number, the claim should be interpreted at that level of precision. DSM also submitted that treating numbers in claims as being precise to the number of decimal places used in the claim has now become an established convention, relying on Smith & Nephew and the subsequent decisions in Napp v Dr Reddy’s [2016] EWHC 1517 (Pat) (Arnold LJ) at [90]-[95], upheld on appeal at [2016] EWCA Civ 1053. In view of DSM’s contention I reviewed these decisions with care.
693. In his first instance judgment in Napp, Arnold LJ reviewed Smith & Nephew and, at [95], discussed how Kitchin LJ applied his approach (summarised at [38]) to the claim in question:
‘…He first rejected an exact value understanding of the figures. The skilled addressee would not understand the patentee to have intended the limits to be read in that way. The question was what degree of precision was required. In that case, the question was whether the skilled addressee would apply a whole number approach or a significant figure approach to the construction of 1% to 25%. The difference between the two was that the whole number approach would cover a range =0.5% to <25.5, whereas the significant figure approach would cover a range =0.95% to =25.5%. He held that the whole number approach was correct, for the reasons he expressed at [60] as follows:
"In my judgment there can be no logical basis for preferring the significant numbers approach over the whole number (or zero decimal places) approach in construing the claim in issue. The purpose of expressing numbers to a particular degree of precision may be to convey to the reader the degree of accuracy with which he needs to make a particular measurement or carry out a calculation. In the context of the claimed method, it is to convey to the reader the range of permissible binding agent concentrations and the accuracy with which those concentrations need to be determined. There is no reason to suppose that this can vary depending upon whether the bottom of the range is 1%, 2% or 5%, or whether 10% is at the top or bottom of the range. It seems to me that Professor Kennedy therefore put it entirely correctly in saying as he did in his first expert report that it is not the number of significant figures that is important in this context, and instead it is the precision with which a number is written. I consider that Professor Kennedy was also right to say that the skilled person would understand the 1% and 25% limits to have been expressed to the nearest whole number."’
694. So, in the context of the patent in Smith & Nephew, the exact value understanding was rejected in favour of the question of what degree of precision was used in the claim, Kitchin LJ concluding that the whole number approach applied, based in part on the expert evidence from Professor Kennedy.
695. In Napp, the issues concerned what was meant by ‘10%-wt buprenorphine base’ and ‘about 10%-wt oleyloleate’. In context there, Arnold LJ decided that the buprenorphine limit was expressed to the nearest whole number, so extended from 9.5 to 10.5%-wt, whereas the word ‘about’ would be understood to give a small degree of imprecision over and above that permitted by normal rounding, allowing a margin of 1% around the figure of 10%-wt.
696. Mara relied on the slightly later discussion of the principles applicable to the construction of numerical limits, as set out by Floyd LJ in Jushi Group Co., Ltd v OCV Intellectual Capital, LLC [2018] EWCA Civ 1416, [2019] RPC 1 at [36]-[39]. As the headnote in the Reports of Patent Cases indicates, Smith & Nephew was considered and explained.
697. As Floyd LJ made clear at [36] & [37]:
i) the normal principles of construction apply, so the meaning and scope of a numerical range must be ascertained in light of the disclosure of the patent, the common general knowledge and all other relevant circumstances.
ii) The Court of Appeal in Smith & Nephew had not been laying down any rule of law as to how numerical ranges should be interpreted in all cases.
698. Accordingly, Mara submitted that Kitchin LJ’s fifth point regarding the number of decimal places or significant figures to which the numerals in the claim appear to have been expressed is only one factor; their meaning depends upon all the circumstances. I agree.
699. The final point I noted from Jushi is a separate point concerning the way in which Floyd LJ dealt with the alleged anticipation argument which required selections from a number of different ranges. He referred to Dr Reddy’s Laboratories, where the claim to olanzapine was not anticipated precisely because the prior art cannot be treated as a disclosure of each and every possible combination encompassed by a generic disclosure of the prior art. That is the usual situation, but Floyd LJ went on to acknowledge in [55] that it depends on the circumstances:
‘55 I would accept that there may be circumstances where a prior disclosure of a numerical range, such as a range of temperatures to be used in a process, may carry with it an implicit disclosure that the skilled person may choose any value within the range. Whether that is so will depend on the disclosure of the document understood with the benefit of the common general knowledge. It is wrong, however, to elevate that possible conclusion into a rule of law, so that every numerical range must be so understood, whatever the context.’
700. Drawing all these points on numerical values together, DSM’s suggestion that there is any sort of ‘general rule’ or ‘convention’ is plainly wrong. What the authorities make clear is that the normal principles of construction apply; and the meaning and scope of a numerical range must be ascertained in the light of the disclosure in the Patent, the CGK and all other relevant circumstances. Furthermore, the reliance on attempted factual analogies is not useful. The notion that a very small number of decided cases establish a convention which applies across the board flies in the face of the general principles established in Smith & Nephew and Jushi.
701. [0001] states:
‘The present invention relates to processes for obtaining a lipid from a cell by lysing the cell, raising a pH of the cell and/or contacting the cell with a salt, and separating the lipid. The scope of protection is defined by the process as set out in the claims.’
702. Thus, it is clear from the outset that the process is for obtaining or extracting a lipid.
703. Under the heading ‘Background Art’, two methods of lipid extraction are described - in the first, the lipid is separated by solvent extraction - typified by the well-known hexane method in [0002]. [0003] refers to lysing a cell in a fermentation broth using mechanical force (e.g. homogenisation), enzymatic or chemical treatment, with the separation of the lipid from the resulting composition using an organic solvent e.g. isopropanol.
704. The problems with these processes are discussed in [0004], including that industrial scale production requires a large amount of volatile and flammable organic solvent, creating hazardous operating conditions, the difficulties in recovering the organic solvent and the costs of the processes.
705. The aim of EP801 is stated in clear terms in [0005]:
‘Therefore, there is a need for a process for obtaining lipids from a cell which does not use an organic solvent. Several processes have been proposed for separating a lipid from a cell without the use of an organic solvent. For example, U.S. Patent No. 6,750,048 discloses an aqueous washing process whereby an emulsion is washed with aqueous washing solutions until a substantially non-emulsified lipid is obtained. However, in some embodiments, this process requires multiple washing steps, which require substantial cost and time. U.S. Patent No. 7,431,952 discloses a process whereby lysed cells are centrifuged to remove cell wall debris and then oils are extracted and purified. However, this process provides a crude oil that requires extensive further purification. Thus, what is needed is a process that does not utilize a volatile solvent to extract a lipid from a cell, and which can be performed using readily available equipment and a minimum number of steps to provide a highly pure lipid.’
706. There is a rather oblique reference in this paragraph to the need to solve the emulsion problem, although it was common ground that the Skilled Team would know that an oil-water emulsion is likely to form when oil is released from the cells through lysis into an aqueous environment. DSM submitted that it is clear from the statement of the invention in [0007], which aligns with claim 1 as granted, that EP801 claims a method of obtaining a lipid from microbial cells, without using an organic solvent, but which nevertheless solves the emulsion problem. That submission rather begs an important question of construction concerning the last phrase in the claim which I address below.
707. [0007] is the first paragraph in the section headed ‘Brief Summary of the Invention’, which continues to [0053]. [0008]-[0052] list a whole series of alternative features ‘in some embodiments’, frequently expressed as very wide ranges: e.g. agitating for 5 minutes to 96 hours.
708. The ‘Brief Description of the Drawings/Figures’ lists 5 figures, the first four of which are flow charts. Fig. 5 illustrates the effect of Alkaline Treatment using EPR Spectroscopy, showing plots of four pH values against time. Each pH value is expressed to two decimal places 5.51, 7.44, 10.46 and 11.95.
709. The Detailed Description of the Invention runs from [0056] (which again aligns with claim 1 as granted) through to [0352]. The parties only found it necessary to refer to a few of these paragraphs, and it is convenient to group them by the issue to which they are relevant. However, the Skilled Team would note that there are a large number of paragraphs each of which discloses a whole series of possible ranges of a relevant parameter, most of the ranges being extremely wide.
710. By way of one example amongst many, in [0077], the % PUFAs in the lipid, ranged from at least 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45% or at least 50% by weight of desired PUFA, similarly for the % by weight of DHA, DPA n-6, EPA, ARA. Similarly too for the anisidine value of the lipid - 26 or less, 25 or less, 20 or less and so on down to 1 or less; and the peroxide value - 5 or less down to 0.1 or less; and the phosphorous content of 100ppm or less, down to 1ppm or less. See also in terms of ranges of numerical values [0110], [0115], [0118], [0124], [0128], [0130]-[0136], [0140]-[0150], [0152]-[0160] etc.
711. The Skilled Team would not, in my view, find these ranges of possible limits at all informative, in the absence of other, more specific teaching. Rather, with their knowledge of how patents are drafted (cf Virgin v Premium Aircraft Interiors [2009] EWCA Civ 1062, [2009] RPC 8 at [15]), they would regard these ranges as devices inserted by the patent agent from which an appropriate selection might be made, depending on the prior art cited by an examiner against the application.
712. In view of the construction issues raised, I refer to rather more passages in the Detailed Description than the parties did in their submissions.
713. [0056] contains another exposition of the process of the invention in the terms of claim 1 as granted. After a further series of paragraphs listing alternative features in wide ranges, the next heading is ‘Overview’ which starts with this (emphasis added):
‘[0086] Generally, the processes of the present invention do not utilize an organic solvent in order to extract or otherwise separate a lipid. Thus, in some embodiments, an organic solvent is not added to a cell broth comprising plant material or fermentation broth comprising a microbial cell, is not added to a cell composition, is not added to a lysed cell composition, or is not added to a lipid during a process of the present invention in an amount or concentration sufficient to extract a lipid. In some embodiments, an organic solvent can be added to a cell composition, a lysed cell composition, or a demulsified cell composition. In such embodiments, the organic solvent is added in a concentration less than 5%, less than 4%, less than 3%, less than 2%, less than 1%, less than 0.5%, less than 0.1%, or less than 0.05% by volume.’
714. After some definitions which I need not set out, the paragraph concludes as follows:
‘An organic solvent as defined herein can be optionally added to a lysed cell composition, for example, as a component of a base and/or a salt for contacting with the lysed cell composition. However, in such embodiments the organic solvent is present in a concentration such that the lipid is not substantially extracted from the cell composition, lysed cell composition, or demulsified cell composition by the solvent (i.e., in a concentration of less than 5%, less than 4%, less than 3%, less than 2%, less than 1%, less than 0.5%, less than 0.1%, or less than 0.05% by volume or weight).’
715. The next section is headed ‘Definitions’ and runs from [0088]-[0107]. The section after that is headed ‘Processes’. [0108] starts with lysing and includes a very general statement (which echoes [0023]) as follows:
‘As used herein, the terms "lyse" and "lysing" refer to a process of rupturing the cell wall and/or cell membrane of a cell. In some embodiments, lysing comprises a process such as: mechanically treating, chemically treating, enzymatically treating, physically treating, or combinations thereof.’
[0114] As used herein, chemically treating includes, but is not limited to, raising a pH of a cell, contacting a cell with a chemical or the like.
716. In this section I cover the disclosure in EP801 concerning the pH, and record the evidence and associated submissions in which various aspects were discussed, noting that of course the correct interpretation of the claim integer in question is a matter for the Court.
717. Mara relied on the references to pH ranges in both [0115] and [0152] but some care is required to put these paragraphs into their correct context. DSM submitted that [0115] does not relate to step (b). To test that proposition, it is necessary to examine some other paragraphs in the specification.
718. It is necessary to start with [0108], the first paragraph under the heading ‘Processes’
‘[0108] The processes of the present invention comprises lysing a cell or cell biomass to form a lysed cell composition. As used herein, the term "cell biomass" refers to a population of plant or microbial cells. As used herein, the terms "lyse" and "lysing" refer to a process of rupturing the cell wall and/or cell membrane of a cell. In some embodiments, lysing comprises a process such as: mechanically treating, chemically treating, enzymatically treating, physically treating, or combinations thereof.’
719. [0114] and [0115] provide as follows:
‘[0114] As used herein, chemically treating includes, but is not limited to, raising a pH of a cell, contacting a cell with a chemical or the like.
[0115] Raising a pH of a cell can include, but is not limited to, adding a base to a cell composition. [reference is made to various bases and their form (e.g. solid or liquid)]. … In some embodiments, the pH of the cell composition is raised to 8 or above, 9 or above, 10 or above, 11 or above, 12 or above, or a pH of 7 to 13, 7 to 12, 7 to 11, 7 to 10, 7 to 9, 8 to 13, 8 to 12, 8 to 11, 8 to 10, 8 to 9, 9 to 12, 9 to 11, 9 to 10, 10 to 12, or 10 to 11.’
720. At all events, it is true that [0115] uses the same language of ‘raising a pH to’ a certain value or above or into a range.
721. [0117] then proceeds to discuss enzymatic lysing. [0118] then defines a ‘lysed cell composition’:
‘[0118] As used herein, a "lysed cell composition" refers to a composition comprising one or more lysed cells, including cell debris and other contents of the cell, in combination with a lipid (from the lysed cells), and optionally, broth that contains microbial cells or plant material.’
722. I need not set out [0119]-[0120], which shed light on the meaning of ‘demulsified cell composition’. There is no definition of that expression, but those paragraphs and [0123] in particular make it clear to the Skilled Team that demulsification does not have to be complete (or anywhere near complete) for the composition to qualify as a ‘demulsified cell composition’. Provided some measure of lipid can be extracted from it, the composition will qualify as a demulsified cell composition.
723. For completeness I set out the [0122] and the relevant parts of [0123]:
‘[0122] In some embodiments, treating a lysed cell composition with a first base breaks up (i.e., demulsifies) an emulsified lysed cell composition. In some embodiments, treating a lysed cell composition with a second base breaks (i.e. demulsifies) an emulsified lysed cell composition. In some embodiments, treating a lysed cell composition with a salt breaks (i.e., demulsifies) an emulsified lysed cell composition. In some embodiments, heating a lysed cell composition breaks (i.e., demulsifies) an emulsified lysed cell composition. In some embodiments, agitating a lysed cell composition breaks (i.e., demulsifies) an emulsified lysed cell composition. In some embodiments, simultaneous heating and agitating of a lysed cell composition breaks (i.e., demulsifies) an emulsified lysed cell composition. In some embodiments, one or more of the preceding treatments breaks up (i.e., demulsifies) an emulsified lysed cell composition.
[0123] The process of the invention comprises raising the pH of the lysed cell composition to demulsify the lysed cell composition. Raising the pH comprises contacting the lysed cell composition with a base. The process of the invention comprises contacting the lysed cell composition with a base to demulsify the lysed cell composition. …
In some embodiments, the pH of a lysed cell composition or a demulsified cell composition is raised a second time. In some embodiments, the second raising of the pH comprises contacting a lysed cell composition or demulsified cell composition with a second base.’
724. [0125] sets out various bases suitable for use with the present invention and states they can be in a solution comprising an organic solvent, ‘such as methanol, ethanol, propanol and the like’. It continues: ‘Thus, a solvent can be optionally present in a base for use with the present invention.’
725. Then [0126] addresses step (b):
‘[0126] Contacting the lysed cell composition with a base raises the pH of the lysed cell composition to 8 or above, 9 or above, 10 or above, 11 or above, 12 or above.’
726. There follow a series of paragraphs in which very wide ranges of values are specified for a variety of parameters.
727. The final paragraph which concerns pH values is [0152] which lists another set of pH targets, this time in respect of the pH to be achieved on addition of the second base. The second base is described as being to:
‘…raise the pH of the treated cell composition or the treated lysed cell composition to 7 or above, 7.5 or above, 8 or above, 8.5 or above, 9 or above, 9.5 or above, 10 or above, 10.5 or above, 11 or above, 11.5 or above, or 12 or above. In some embodiments, a treated cell composition or a treated lysed cell composition is contacted with a second base to raise the pH of the treated lysed cell composition to 7 to 13, 7 to 12, 7 to 11, 7 to 10, 7 to 9, 7 to 8, 7 to 7.5, 7.5 to 8, 8 to 13, 8 to 12, 8 to 11, 8 to 10, 8 to 9, 8 to 8.5, 8.5 to 9, 9 to 12, 9 to 11, 9 to 10, 9 to 9.5, 9.5 to 10, 10 to 12, or 10 to 11.’
728. Again, [0152] again uses the same language of raising a pH to a value or above or into a range.
729. Mara cross-examined Mr Dueppen on the ranges in [0115] (see [719] above). Mara submitted that ‘7 to 13’ in this list would not be understood as including pH 6.5, since the teaching of EP801 is to use basic conditions (Dueppen XX [T3/354/20 - 355/14]). Mara submitted that the same logic must apply to all of the possibilities that specify a lower limit of pH 7. DSM’s construction would therefore already involve the skilled person, in respect of a single list of possibilities, not reading 7 as including 6.5, but reading 8 as including 7.5. Mara submitted this already lacks coherency, but that DSM’s argument gets worse.
730. Turning to [0152], Mara submitted this list includes not just targets where the pH is stated as an integer, but also targets where the lower limit ends in .5. Mara invited consideration of the two adjacent possibilities ‘8.5 or above’ and ‘9 or above’. Mr Dueppen was compelled to agree that ‘9 or above’ means exactly that, and includes nothing below 9 (Dueppen XX [T3/355/15 - 359/11]). Plainly it would be technical nonsense if both possibilities meant the same thing, i.e. ‘8.5 or above’, or if the particular value was interpreted as meaning the previously stated value.
731. Mara submitted the same must be true of ‘8 or above’. Mr Dueppen confirmed in context it does not mean 7.5 or above, or include anything below 8 (Dueppen XX [T3/359/12-19]).
732. Mr Dueppen further agreed that it would be understood that, since the pH range is divided up into discrete and adjacent half-integer target ranges (7 to 7.5, 7.5 to 8, 8 to 8.5 etc), each of these would be understood to mean what it says - so a target of 8 to 8.5 requires a pH within that range, and not one down to 7.5 (Dueppen XX [T3/359/20 - 360/18]). Mara therefore contended that these ranges would not be understood as ranges in which different rounding conventions apply at each end of the range. On this point, I agree that Mr Dueppen struggled to avoid agreeing (Dueppen XX [T3/360/19 - 362/18]), even though DSM attempted to put a different explanation forward: that Mr Dueppen’s difficulties arose from the conflict between ‘the detailed linguistic analysis being conducted on paragraph [0152] and the reading of the scientist’. However, what came through clearly was that Mr Dueppen viewed these paragraphs at an inappropriately high level of generality: that EP801 was simply teaching that an alkali pH can be used.
733. As Mara submitted, one point that Mr Dueppen could agree was that a measured pH of 7.8 would fall within the target 7.5 to 8, and not the target 8 to 8.5 (Dueppen XX [T3/362/19-23]). Mara submitted that this is consistent with Mr Dueppen having described his example target of pH 6.9 to 7.3 being a range of 0.4 pH units, and not a range of 0.5 units, as it would be if conventional rounding were applied to the limits (Dueppen XX [T3/362/24 - 363/19]).
734. The only point that was sought to be made in XX of Dr Kyle was to point to one Mara process that used a (confidential) target range with a width of 0.5 pH units, with the suggestion that it was not necessary for Mara to be more precise than this in their process (Kyle XX [T6/847/14 - 849/5]). Mara submitted that this does not begin to meet the point because EP801 has many target ranges that have a width of 0.5 pH units. Instead, Mara submitted that the question at stake is, how are the limits of those target ranges to be understood? And are the targets really of width of 0.5 units, or are they much broader (e.g. ‘9 to 9.5’ actually meaning ‘8.5 to 9.5’, or perhaps ‘8.5 to 9.55’ on DSM’s approach)? Mara submitted the answer was clear: plainly not, as Mr Dueppen agreed. Whether in a particular process the target range is chosen to be 0.5 units wide (e.g. ‘9 to 9.5’) or 1 unit wide (e.g. ‘9 to 10’), or some other width (e.g. ‘9 or above’), does not inform construction of the numerical limits.
735. Mara submitted that all this points only one way. EP801 must be understood as specifying targets where the limit is strict. A target pH of ‘8 or above’ means the pH cannot be below 8.
736. DSM proposed in opening that the numbers in the claim are to be taken to be expressed in claims at the level of precision at which they are expressed. However, Mara’s response was that this ignores the law as summarised in Jushi that the normal rules of construction apply to numerical limits, and that scope of a numerical range must be ascertained in the context of the specification. When this is done, Mara say there are overwhelming reasons to reject DSM’s construction.
737. Before analysing these and other points, in view of DSM’s ‘level of precision’ argument, it is necessary to have regard to other references to pH levels in EP801. DSM drew particular attention to Examples 17 and 18, but I prefer to refer to a few more.
738. The Examples are prefaced with [0204]:
[0204] Having generally described the invention, a further understanding can be obtained by reference to the examples provided herein. These examples are given for purposes of illustration only and are not intended to be limiting. The following examples are illustrative, but not limiting, of a process and a lipid prepared by a process of the present invention.
739. The Examples fall into at least the following categories:
i) Examples 1, 3, 4, 5, 6, 16, 18, 19, 21, 25 & 39 feature the use of Alcalase i.e. enzymatic lysing.
ii) Examples 2, 7, 8, 17, 20 & 22 feature homogenisation i.e. mechanical lysing.
iii) Examples 9-15 feature chemical lysing, using a base - in each case a 50% solution of NaOH.
iv) The remaining examples are either comparative or report results of particular testing (e.g. sensory) or further analysis of earlier Examples. Thus, Examples 23-27 involve various comparisons between lipids obtained from one of the earlier examples and either a hexane extraction or a FRIOLEX process. Example 28 involves a comparison with a prior art process for extracting lipids without the use of an organic solvent. Examples 29 and 30 concern the characterisation of the particular thraustochytrid PTA-9695 as a new Schizochytrium species, with Example 30 including the various tables of Fatty Acid Profiles
740. There are various references to pH in the Examples. It is unnecessary to detail all of them as the general picture emerges from the following extracts. I will take the different methods of lysis in turn.
741. So far as enzymatic lysis is concerned, it suffices to quote the following reports of pH:
i) Example 1: ‘A second base (NaOH, 50% w/w solution,40 kg) was then added to the lysed cell composition until the pH was above 8.’ And ‘The pH of the lysed cell composition was maintained at 7.5 to 8.5 during the centrifuging.’
ii) Example 4: ‘…treated with a first base… to adjust the pH of the lysed cell composition to 10.5’. ‘A second base was added …until the pH was 8.3’
iii) Example 5: ‘…to adjust the pH ….to 10.5’. ‘[held at 95°C plus agitation for 1 hour] and the pH dropped to 8.5.’
iv) Example 6: ‘…to adjust the pH ….to 10.62’. [various actions performed] ‘…until the pH was 8.13…9.02….10.12.’
v) In many of these examples, the first base was added to adjust the pH of the lysed cell composition to 10.5.
vi) Since DSM placed specific reliance on Example 18, I will quote the relevant parts. Following the addition of Alcalase ‘to lyse the cells and form an emulsified lysed cell composition, it is stated:
‘The emulsified lysed cell composition was treated with a base (i.e., a 12.5% solution of NaOH) to adjust the pH of the
lysed cell composition from 7.21 to 10.52.’
‘The broth was then separated into 4 portions … The individual trials were then centrifuged without further pH adjustment.’
The pH for each centrifugation is recorded in Table 2 as 6.22, 8.19, 8.38 and 10.03.
vii) In closing, DSM also referred to Examples 19 & 21. I am not at all sure they take matters any further but I will briefly describe them. Example 19 used Alcalase and the addition of a base to raise the pH from 6.8 to 10.6. After the addition of a salt, heating and a hold time, the broth was separated with one half being centrifuged as is and the other after adjustment of its pH to approximately 8.5. The results are shown in Table 3, but the AV values were higher than expected, probably because the lysed cell composition was allowed to sit for a long period of time before the lipids were extracted, leading to oxidation. The pH values for the as is samples were 6.0, 5.5 & 5.7, and the others were 8.4, 8.4 and 8.5.
viii) Example 21 used a sample of Alcalase enzyme treated lysed cell composition obtained from Schizochytrium cells, which had a pH of 5.5. It was divided into four and three of the samples were adjusted to a pH of ‘approximately 7.4, approximately 10.5 and approximately 12’. Measurements were taken by EPR spectrometer of the amount of free radicals present from lipid oxidation and the readings are shown in Fig 5. The data show the advantage of a higher pH, demonstrating the addition of a base inhibits lipid oxidation.
742. All the enzymatic lysis examples use Alcalase 2.4L FG 0.5% but one feature of note is that the pH of the enzymatic lysing step is not reported. A pH value is generally only reported after the addition of a base. Example 21 was an exception but it would appear the reported pH of 5.5 was after lysis had completed, or substantially so. The Skilled Team would understand that lysis would cause a drop in the pH.
743. So far as mechanical lysis is concerned:
i) Example 2: ‘The lysed cell composition was treated with a base (i.e., NaOH, 10 g of a 50% w/w solution) until the pH of the lysed cell composition was 10.4 to 10.6.’ ‘The pH of the lysed cell composition during centrifuging was maintained at 6.5 to 8.5.’
ii) Example 7: ‘…to adjust the pH ….to 10.5’. [various actions performed] ‘…until the pH was 8.07…9.11….10.09.’
iii) Example 8: ‘…the pH was adjusted to 10.5’. [salt added plus heating] ‘…and the pH had dropped to 9.5 after 1-2 hours….’
iv) Since DSM placed particular reliance on Example 17, I set it out here:
‘The lysed cell composition was treated with a base (i.e., a 12.5% solution of NaOH) until the lysed cell composition reached a pH of 7.8 to 8.2. A salt (solid Na2SO4, in an amount of 5% by weight of the lysed cell composition) was added to the lysed cell composition. The lysed cell composition was then heated to a temperature of 60° C and held at that temperature. The pH of the lysed cell composition was maintained at the 7.8 to 8.2 level by the addition of base…’
‘This resulted in an oil layer of about 2 ml in a sample of 40 ml. The extraction yield of the oil was 73% by weight.’
744. So far as the Examples involving chemical lysis are concerned, although the actual pH readings vary, each of these Examples feature common wording (emphasis added):
‘The broth was chemically treated with a base (i.e., a 25% solution of NaOH) without a prior cell lysis step. The addition of the base raised the pH of the broth from 5.8 to 11.2. The addition of the base and the rise in the pH lysed the cells to form a lysed cell composition.’
745. In Examples 9, 10 & 15, the next step was the addition of a salt (either Na2SO4 or NaCl), following by heating to a particular temperature and held there for a specified time. In Examples 11-14, no salt was added but heating took place, again to a particular temperature and held there for a period of time. Where a resultant pH value was reported, it was to the effect that the pH had dropped to a particular value: pH 9.7 [9], 8.7 [10], 10.36 [11].
746. The principal claim in issue is claim 1A, which has the added integer limiting the method of lysis to enzymatic. Claim 1 as granted covered all methods of lysing.
‘1A. A process for obtaining a lipid from a microbial cell, said process comprising:
a) lysing a cell to form a lysed cell composition;
b) adding a base to the lysed cell composition, raising the pH of the lysed cell composition to 8 or above to demulsify the cell composition;
c) one or more of c1, c2, c3 and c4;
c1) adding a salt to the lysed cell composition to demulsify the cell composition
c2) heating the lysed cell composition to demulsify the cell composition
c3) agitating the lysed cell composition to demulsify the cell composition
c4) adding a second base to the lysed cell composition to demulsify the cell composition;
and
d) separating a lipid from the demulsified cell composition;
wherein the lipid contains less than 5% by weight of an organic solvent and wherein the lysing comprises enzymatic treatment.’
747. DSM also relied, as necessary, on claims 6A and 7A, which are dependent on claim 5A:
‘5A. The process according to any preceding claim, wherein the process comprises heating the lysed cell composition to demulsify the cell composition.
6A. The process according to claim 5A, wherein the heating is performed after the adding a salt.
7A. The process according to any preceding claim, wherein the process comprises agitating the lysed cell composition to demulsify the cell composition.’
748. By the time of closing argument, a number of construction issues had been raised. I propose to resolve the more minor issues before addressing the more significant issues which arose on ‘raising the pH of the lysed cell composition to 8 or above to demulsify the cell composition’ and DSM’s related argument concerning the ‘order of steps’.
749. At one stage it seemed that there would be a dispute over the meaning of the term “lysis” and cognates such as “lysed”, but in fact there seems to be agreement on the point. EP801 defines “lyse” and “lysing” at [0108] as meaning “a process of rupturing the cell wall and/or cell membrane of a cell”. The experts were agreed that lysis would be understood as meaning sufficient degradation of the cells to allow intracellular oil to be released.
750. As for “a lysed cell composition”, I have set out the definition of this term in [0118] in [721] above.
751. DSM acknowledged that this could be read literally as requiring only a single cell to be lysed, but submitted that a more practical approach is required. DSM submitted that the experts agreed that the term used in claim 1A would be understood as requiring (at least) sufficient cells to be lysed and therefore sufficient oil to be released to form an emulsion, as contemplated by the remainder of the claim.
752. Whilst I acknowledge the practical approach suggested to and agreed by the experts, they are not experts in the interpretation of patents. In this regard, it is relevant to note that the definition of a ‘lysed cell composition’ is extremely broad. In other words, as soon as some degree of lysis has started producing some level of lipid, there is a lysed cell composition. There would also likely to be some degree of emulsion.
753. For completeness I mention that in his first report, Dr Kyle went further and argued (evidently, as DSM submitted, for the purpose of the non-infringement case, no longer pursued) that in claim 1A “a lysed cell composition” would require “most, or substantially all, of the cells [to] have undergone lysis”. I leave his point out of account.
754. DSM argued that EP801 clearly distinguished between ‘a cell composition’ and ‘a lysed cell composition’. Even if I assume that ‘a cell composition’ means that no cells have been lysed, the difference between the two expressions is small, bearing in mind the breadth of the meaning of ‘a lysed cell composition’.
755. It is true that (i) the specification frequently refers to an action on ‘a cell composition or a lysed cell composition’ and (ii) there is no definition of ‘a cell composition’ but, as Mara pointed out, claim 1A does not appear to conform to the supposed distinction, using ‘lysed cell composition’ in steps (a) and (b), but ‘cell composition’ later in step (b) and (d) but both expressions in (c1) to (c4). In response, DSM submitted there was nothing wrong with this usage.
756. The debate was verging on meticulous verbal analysis. Overall, the exercise seemed to me to be particularly pointless in view of the almost vanishingly small difference between the two in view of the breadth of ‘lysed cell composition’. However, I will have to return to this point when I consider the ‘order of steps’ argument.
757. As DSM submitted, these words comprise a functional limitation. They appear in step (b) and each of steps (c1) to (c4). DSM made the following points.
758. First, the degree of demulsification required. DSM said this related to sufficiency rather than any issue on infringement. [0119] says this:
As used herein, the terms "emulsion" and "emulsified" refers to a mixture of two or more immiscible phases or layers wherein one phase or layer is dispersed in another phase or layer. As used herein, the terms "break," "break up," "demulsify," "demulsification," "demulsifying," and "breaking" refer to a process of separating immiscible phases or layers of an emulsion. For example, demulsifying or breaking an emulsified lysed cell composition refers to a process by which an emulsified lysed cell composition changes from an emulsion having one or more phases or layers to a composition having two or more phases or layers. For example, in some embodiments, a process of the present invention breaks an emulsified lysed cell composition from a single-phase to two or more phases. In some embodiments, the two or more phases include a lipid phase and an aqueous phase. In some embodiments, a process of the present invention breaks an emulsified lysed cell compositions from one or more phases to at least three phases. In some embodiments, the three phases include a lipid phase, an aqueous phase, and a solid phase. In some embodiments, the three phases include a lipid phase, an emulsion phase, and an aqueous phase.
759. DSM submitted that this makes clear that the term does not require every droplet of oil to come out of the emulsion, because it includes within the definition the case where a single-phase emulsion is broken into a three-phase composition including oil, water and residual emulsion (see the final sentence).
760. At the opposite end of the scale, if all or virtually all the oil remains in the emulsion, DSM were prepared to accept that there has been no demulsification.
761. On that basis, DSM submitted the only sensible reading of the claim is therefore that it requires a significant amount of oil to be separated from the emulsion, so that it can be recovered at step (d) of claim 1A. This is another gloss on the meaning of the claim. The claim does not require the recovery of a significant amount of oil from the lipid, just some.
762. Again, for completeness I mention Dr Kyle’s suggestion that the claim requires “a significant majority” of the emulsion being broken down. DSM said this was incompatible with [0119] and makes no technical sense - why would the patentee want to exclude a process in which a significant amount of lipid is recovered? In any event, Mara did not seek to defend Dr Kyle’s construction, having abandoned the non-infringement argument that it would have given rise to.
763. Second, the words “to demulsify the cell composition” when used in relation to a step do not require that step to demulsify the cell composition immediately or without other steps being taken. DSM suggested that follows from the fact that claim 1A clearly contemplates a combination of steps which collectively have the effect of demulsification, without any one of them necessarily doing it on its own. Indeed, they said that if the pH raise to 8 or above at step (b) immediately and completely demulsified the cell composition, then it would be difficult to see how step (c) could ever be performed.
764. Accordingly, DSM submitted that the claim is satisfied if step (b) and at least one of steps (c1) to (c4) together contribute to demulsification in a material way. DSM submitted that this appears to be common ground in light of Mara’s acceptance of DSM’s factual case on infringement. In any event, I agree with this point.
765. Third, it was common ground that there is no requirement of intention. The integer is satisfied if the effect of the relevant steps is to contribute to demulsification in fact. Again, I agree.
766. In opening, Mara pointed out that the integer requiring that “the lipid contains less than 5% by weight of an organic solvent” does not require that the process is done without solvent, or with less than a particular level of solvent (or even that there is less than 5% of organic solvent present after step (d)). The claim is to a process comprising steps (a) to (d). It does not preclude further steps, including in particular a step of solvent removal prior to step (d). Accordingly, so Mara submitted, a process will satisfy this integer if, after use of solvent, and removal of solvent, there is less than 5% of organic solvent remaining in the lipid.
767. DSM proposes, by contrast, that this integer “avoids organic solvents” (DSM’s EP801 skeleton ¶44), which appears to suggest that the process of claim 1 cannot include solvents (or perhaps cannot include their use at >5%). DSM referred to two paragraphs of the evidence, which Mara commented on as follows:
i) Dueppen 1 ¶178. Here Mr Dueppen simply gave his view of construction based on the claim language (as set out in [0007]), but construction is not for the experts.
ii) Kyle 1 ¶346, where Dr Kyle was referring to [0075] and [0086]. [0075] says that in some embodiments, the process does not add an organic solvent to the lysed cell composition. That sheds no light on the claim language. [0086] contains some teaching about what proportions of organic solvent may, in some embodiments, be added to the lysed cell composition (one suggestion being that the organic solvent is added in a concentration less than 5%), and Dr Kyle commented on this aspect. However, that is not what the claim is about - there is no step (c5) of “adding an organic solvent in a concentration of less than 5% to the lysed cell composition to demulsify the lysed cell composition”.
768. For those reasons, Mara submitted that the evidence that DSM relied on is not material to construction of this integer. The integer is about the solvent content of the final lipid, and is incapable on the normal rules of construction of having the meaning that DSM attributes to it.
769. DSM argued that this integer would be understood to mean an aqueous extraction process which avoids organic solvents. By contrast, as I have said, Mara argued that this integer refers to the lipid at the end of the process, with the effect that the claim includes processes which use any amount of hexane, isopropanol and/or any other organic solvent - just so long as the lipid is separated from the solvent at the end. On this basis, Mara contended that claim 1A covered a process using solvents (i.e. a modified version of Example 3 of Kobzeff).
770. DSM expressed surprise at Mara’s argument and, in closing, submitted it was clearly wrong, relying on the following points:
i) First, DSM contended there is no question that EP801 is directed at a solventless process, and it would be utterly absurd to construe claim 1A as covering one which uses organic solvent to extract or separate the lipid. The hexane method and the FRIOLEX method are both acknowledged prior art at [0002] and [0003]; and then [0005] states in terms that the difference between those processes and the process of the invention is that the invention does not use organic solvents: “there is a need for a process for obtaining lipids from a cell which does not use an organic solvent”. The solventless solution, corresponding to the granted claim, is then set out at [0007].
ii) Second, DSM contended the experts agreed with their construction, relying on these answers which Dr Kyle gave in cross-examination (also saying that Dueppen 1 ¶178 was to similar effect):
Q. What we see on this page is current method, to use solvents; there is a need to avoid solvents; here is my method that does not use solvents; yes?
A. Yes.
Q. This is how I am going to do it without solvents; yes?
A. Yes.
iii) Third, DSM contended the point is made even more explicit in the Overview at [0086]: “Generally, the processes of the present invention do not utilize an organic solvent in order to extract or otherwise separate a lipid”. It explains that if an organic solvent is added, it is at a concentration of less than 5% such that “the lipid is not substantially extracted from the cell composition, lysed cell composition, or demulsified cell composition by the solvent”. DSM submitted that this is express teaching that solvent is not present at ≥5% concentration at any stage in the process.
iv) Fourth, DSM pointed out that a number of examples (e.g. Examples 23, 24 and 25) use hexane and/or FRIOLEX extraction as a comparator to the solventless extraction of earlier examples in EP801.
v) Fifth, DSM submitted that the key to resolving the construction issue is to recognise that claim 1A is a process claim, and this integer is a characteristic of that process and not merely the end product. The parts of the specification quoted above focus on the process not using solvents, they are not about avoiding having any solvents in the end product.
vi) It follows, so DSM submitted, that a process in which, during the process, more than 5% organic solvent is mixed with the lipid, would not have the specific characteristic. In other words, this integer applies throughout the process and requires a substantial absence of solvent throughout.
771. DSM concluded by saying:
i) Their construction is the only one which makes sense and is consistent with the insistence in EP801 that it is disclosing and claiming a solventless process.
ii) Mara’s construction would render this integer meaningless, because every process involving organic solvents involves removing the solvent from the lipid by the end of the process.
Analysis
772. Despite DSM’s impassioned submissions and despite the stated aims of EP801, the correct interpretation of this integer is, in my view, clear. The requirement that there is less than 5% by weight of an organic solvent applies to the lipid. The lipid is plainly the end result of the process in the claim. Furthermore, because the process is expressed as comprising the specified steps, it does not exclude other steps such as the addition and removal of e.g. organic solvent.
773. I take all of DSM’s points about the teaching in the specification and the use of processes involving isopropanol and the FRIOLEX process as comparators and it is true that the preferred embodiments either have no organic solvent or less than 5%.
774. However, it would have been relatively straightforward for the patentee to have written an integer in the claim limiting or even eliminating the use of an organic solvent throughout the process, to reflect the teaching in the specification on which DSM placed such heavy reliance. For example, ‘wherein throughout the process the cell composition or lipid contains less than 5% by weight of an organic solvent’ or the proposed step c5 (see [767.ii)] above).
775. I take DSM’s point that they wanted to avoid a potential infringer escaping infringement of their solventless process by adding a small amount of organic solvent, but that could have been achieved, as my wording demonstrates. Instead, the patentee chose to impose the limit only on the lipid which is the output of the claimed process.
776. As is trite, the claim is expressed in words of the patentee’s own choosing. DSM cannot escape the plain meaning of this integer. By contrast, DSM’s construction would require the Court to rewrite the claim and that is not permissible.
777. The construction issue identified by the parties on this integer concerned the numerical limit. However, another important issue involved ‘raising’ and the order of the steps argument. I will deal with each issue in turn.
778. DSM said the construction of this numerical limit relates to infringement of some Mara processes.
779. DSM submitted that the numerical pH limit ‘8 or above’ should be interpreted as covering pH values of 7.5 or above. DSM were keen to stress however that their case was really that the claim should be construed so that it covers all values which are 8 or above when those values are stated as a whole number. Thus, for example, the reason why a pH value of 7.8 falls within the claim is because, when that value is stated with the same degree of precision as the claim, that value is 8. So DSM characterised the issue as concerned with the degree of precision with which the number 8 is stated in the claim.
780. For their part, Mara contended that, in context, ‘8 or above’ meant just that. In other words, 8 was a bookend and any value below 8 did not fall within the claim.
781. Inevitably, I made reference to certain of the parties’ submissions when discussing the evidence above. I confirm I have those points well in mind. I have discussed the relevant principles above and, again, I have them well in mind.
The submissions in more detail
782. DSM put forward a range of positive reasons to follow what they suggested was ‘the general rule’:
i) First, DSM suggested that the CGK was that in processes of this sort, more precise pH control than to a whole number is not necessary. DSM indicated this was one answer to Mara’s point about pH being a logarithmic scale. DSM said this point was established by two pieces of evidence. First, Dueppen 2, 154, but in that paragraph what he said was ‘…in real world processes the pH is often not controlled to within anything close to 0.1 units. Indeed in Process 13 itself the target pH is a spread of 0.5 units’. Second, some cross-examination of Dr Kyle on the same feature of Process 13. Reference to what Mara does in 2023/2024 is hardly compelling evidence of CGK in 2010. In any event, DSM’s argument seems to me to elide a number of issues: one is measurement of pH, which EP801 itself establishes can be measured to two decimal places, albeit an instantaneous measurement; a second is a target pH; and the third is the extent to which pH may vary over time within a process.
ii) In a number of passages, EP801 identifies broad ranges of pH values in the alkaline range. [0115] and [0152] were addressed with Mr Dueppen in cross-examination, who was clear that the skilled person would understand the paragraphs as a whole to be teaching that alkaline pH can be used. This was, however, something of a generalisation.
iii) DSM singled out Example 17 of EP801 as ‘illustrative of the lower end of the claimed range, and uses a pH of “7.8 to 8.2”’.
iv) As Mara pointed out, the specification sometimes denotes a pH value to one decimal place. In fact, sometimes it is denoted to two decimal places (e.g. Example 18). Moreover Example 19 includes a “6.0”. As the Court of Appeal pointed out in Smith & Nephew v ConvaTec [47] & [61], this is a positive reason in favour of treating the claim as being expressed to the nearest whole number, because: “this shows is that the author knew full well how to express numbers with different degrees of precision, and that when it came to the claim, he chose limits expressed to an accuracy of zero decimal places”.
783. DSM also contended that it was important to note that ‘when the Court follows the general rule, it is not really extending the claimed range (although the result is sometimes expressed in that way). Kitchin LJ’s words at [38] in relation to this scenario were carefully chosen and bear repeating: “the skilled person would understand that the patentee has chosen to express the numerals in the claim to a particular but limited degree of precision and so intends the claim to include all values which fall within the claimed range when stated with the same degree of precision”. So for example, in FNM Corporation v Drammock, 45.4% did not exceed 45% because when the former number was stated at the correct degree of precision, it was 45%.’
784. So far as the content of the specification is concerned, DSM raised various disputes on Mara’s approach which I have discussed above. DSM’s positive case, as I understood matters, focussed particularly on Example 17, submitting that it was an example where the lower end of the range in the claim was being used, and an indication that a strict cut-off is not necessary.
785. I agree that the pH values in Example 17 can be taken as such an indication. However, those pH values are equally consistent, in my view, with the operator aiming specifically for a pH of 8 (as opposed to 8 or above).
786. Overall, I conclude that DSM place far too great an emphasis on just one example in the specification. What is clear from Example 17 and the various other pH values I have quoted is that instantaneous pH values can be and are measured to one or even two decimal places, but that pH values in these sorts of processes may be varying all the time as processes continue. Thus, whilst it appears possible to measure pH to one or two decimal places at a particular moment in time, the patentee in EP801 appears, very sensibly, to have stated target pHs at a much more general level - either to whole number pH values or, at most, to 0.5 increments. Thus the fact that measurements of pH in the Examples were stated to one and two decimal places does not assist in the interpretation of target values.
787. Furthermore, on this issue there was, in my view, a good deal of meticulous numerical analysis. A simpler purposive approach is required to target pH values. In my view it is plain that all the target pH ranges in the specification must be read in the same way and consequently, it is not possible to interpret any of the whole number pHs quoted as including the value 0.5 units below - see [0152]. Although that paragraph addresses the addition of a second base, no reason is given or has been identified why the pH must be controlled more precisely in that step as opposed to step (b).
788. The final important piece of context is that step (b) says ‘raising the pH to 8 or above’. If the target pH was stated as simply 8, I could see a good argument for interpreting ‘8’ as indicating a range around that figure - say 7.5-8.5. However, the word ‘raising’ suggests a movement of the pH so that it is above 8. The Skilled Team would understand this suggestion, since they would expect lysis to have the effect of lowering the pH of the biomass.
789. DSM’s construction is that ‘raising the pH to 8 or above’ includes raising the pH to 7.5 on the basis that 7.5 means 8. However, if the patentee had wished to claim a pH of 7.5 or above, it could have said so and, in my view, it would have said so.
790. For all these reasons, in the context of this specification, the target pH value of 8 or above means exactly that: 8 or above.
791. In case I am wrong on this issue, I will also consider the effect of DSM’s construction that the claim encompasses a pH of 7.5 or above.
792. DSM were keen to emphasise that the claims consist of both functional and structural features. DSM submitted that features such as: “lysing a cell”, “adding a base”, “adding a salt” are structural features. They each specify a physical step which the implementer has to carry out.
793. On the other hand there are functional features in the claim, in particular: “to demulsify the cell composition”. Features such as this specify the functional effect of the structural features. DSM submitted that the fact that the claim requires steps (b) and (c) actually to demulsify the cell composition is reinforced by the language of step (d), which refers to “the demulsified cell composition”.
794. DSM submitted this is important because it means that a process comprising the structural features, but which does not achieve the specified functional effect, is outside the claim (cf FibroGen v Akebia [2021] EWCA Civ 1279, [2022] RPC 7, [110] (Birss LJ), [288] (Sir Christopher Floyd)).
795. I found this a surprisingly desperate submission from DSM, for the following reasons.
796. EP801 contains no definition of a ‘demulsified cell composition’ and furthermore, as I explained above, the specification makes it clear to the Skilled Team that, in essence, any degree of demulsification will qualify. So DSM’s attempted factual analogy with Fibrogen just does not work. In the paragraphs cited from Fibrogen both Birss LJ and Floyd LJ pointed out that it was wrong (in that case) to say that the structural features are all that is required to achieve the claimed therapeutic efficacy. In this case, it is clear that the structural features of the claim are taught as contributing to demulsification, but those structural features (whether singly or collectively) did not have to achieve complete demulsification. To my understanding, the idea of achieving complete demulsification is probably a pipe-dream and/or impractical.
797. DSM’s next point was that claim 1A specifies that steps (b) and (c) have to be after (a), but before (d).
798. The significance of this is that DSM contended that raising the pH of the broth to 8 or above (or carrying out any of the (c) steps) is not within the claims unless there has already been a substantial degree of lysis, so that the composition can properly be described as a “lysed cell composition” before that step is taken. In other words, raising the pH of the broth to 8 or 9 does not satisfy step (b) if, at the time that is done, the broth cannot properly be described as a “lysed cell composition”.
799. DSM stressed that the pleadings, evidence and submissions of both parties have always proceeded on the basis that this is the correct construction, a point I do not find persuasive.
800. On the other hand, DSM submitted that it is clear from the wording of the claim that the various steps of (b) and (c) can be done in any order (save that, as a matter of logic, step (c4) has to come after (b)).
801. In my view, DSM’s submissions were based on a far too literal approach to the specified process steps, divorced from the approach of the practical Skilled Team. The Skilled Team would be well aware that the process steps specified in claim 1A are not distinct.
802. Furthermore, DSM’s submission here conflicts with their submission on the interpretation of ‘lysed cell composition’ and with my finding on the proper interpretation of that expression - see [752] above. It is worth just spelling out the consequences of that interpretation. Step (a) lysing a cell to form a lysed cell composition can be said to be satisfied by a wide range of actual lysis having occurred - for the sake of argument let me assume a range from 5% lysis to 95% lysis, although in view of the definition of lysed cell composition, there is no reason to exclude percentages even lower than 5%. The same must be true in step (b). This has two consequences: first, that lysis in step (a) does not have to be complete or even near complete before step (b) and second, there can be a good deal of overlap between processes of lysis and demulsifying. Steps (a) and (b) may well overlap to a considerable extent.
803. If the pH of the biomass is below 8, then the addition of base in step (b) will raise the pH to 8 at a definite moment in time, even if the rate of increase of pH is gradual (as is likely to be the case in large reaction vessels). The other point to bear in mind, which the Skilled Team will be well aware of, is that the process of demulsification does not switch on when the pH reaches 8 or above. It is very likely to start at a lower pH before it reaches what might be assumed as the optimum demulsification conditions at pH 8 or above. These practical considerations again confirm that lysis and demulsification will overlap to a considerable extent. Furthermore, that there can be or is significant overlap makes technical sense: the process of demulsifying the emulsion can take place at the same time as the continuation of lysis and formation of additional emulsion with the newly released oil. I recognise that in a practical process, adequate time might well be allowed for lysis before demulsification started, but that does not rule out overlap.
804. What this means is that the practical Skilled Team would not understand the claim as specifying DSM’s strict order of steps. Of course, there are some logical constraints on the order of steps:
i) step (c4) cannot occur before step (b).
ii) at least some lysis has to occur before any lipid can be obtained.
iii) it may be that some demulsification must occur to obtain separation of the lipid from the demulsified cell composition, although that depends on the degree of emulsification.
805. Those aside, in my view, the Skilled Team would have no trouble at all contemplating lysis and demulsification occurring at the same time. To that end, no technical reason was identified as to why any of (c1) to (c3) had to occur after (b). Those were all options to aid demulsification.
806. Although the amended claim 1A focusses attention on enzymatic lysis, it should not be forgotten that claim 1 as granted covered all methods of lysis.
807. I have summarised above the various examples and it seems clear that Examples 1-22 at least were presented to the Skilled Team as examples of the invention in claim 1 as granted. It is worth concentrating for a moment on the chemical lysis examples. I have drawn attention to the common wording used in those Examples - see [744] above. It is clear that in those examples, lysis is attributed to the addition of a base and the rise in pH. There is no further addition of a base. In my view, these examples demonstrate clearly that DSM’s ‘order of steps’ argument is wrong.
808. Against that backdrop, the Skilled Team would understand that there is no magic or technical purpose which requires the strict order of steps for which DSM argued. This also impacts on the scope of protection around the word ‘raising’. In my view, the word was being made to do far too much in DSM’s argument and they attribute far too much significance to it. The wording of the claim reflects the likely position where either the starting biomass is acidic or (to the same effect) lysis causes the pH to drop so that in most, if not all cases, the pH will have to be raised to 8 or above, and that is its true significance. However, as Mr Dueppen explained (see [677] above) what has technical significance is the state of being at an elevated pH of 8 or above, not the changing of the pH. I realise that he gave that evidence in the context of DSM’s equivalence case but this issue seems to me to affect both normal interpretation and equivalents.
809. Where the dividing line between the two arguments appears to me to be as follows. It would be illegitimate to write out from the claim the word ‘raising’, so the claim requires raising of the pH to 8 or above. However, in my view, this element of the claim is satisfied if the pH is raised to 8 or above at any point in the process. The consequence is that if the pH is above 8 but then drops because lysis continues, this element of the claim is satisfied if base is added to raise the pH to 8 or above.
810. On DSM’s alternative construction, all these considerations apply to the pH limit of 7.5.
811. Before I analyse the prior art, it may assist to have in mind a key argument of Mara on validity which focuses on the so-called bonus effect. In this case, the bonus effect is said to arise from carrying out a process at a pH which Mara say is obvious to use and the bonus effect being assisting with demulsification. Mara’s obviousness challenge was said to engage the principle that workers in the art are allowed to take obvious steps and if, in doing so, they hit on something useful, then that is a bonus that they are entitled to take without fear of being sued for infringement of a patent.
812. In Actavis v ICOS at [73] [JA1/1/28] Lord Hodge explained by reference to Hallen v Brabantia that an invention that is obvious for one purpose is not saved by a bonus effect in respect of a different purpose:
‘73. Ninthly, it is necessary to consider whether a feature of a claimed invention is an added benefit in a context in which the claimed innovation is obvious for another purpose. In Hallen & Co v Brabantia (UK) Ltd [1991] RPC 195 the Court of Appeal was concerned with an alleged selection patent for a self-pulling corkscrew which had a helix coated with polytetrafluoroethylene (PTFE) which was a known friction-reducing material. At the priority date PTFE had been used for several years to coat the helix of a twin-lever type corkscrew to aid its penetration into the cork. The PTFE-coated helix had this effect also on the self-pulling corkscrew, a fact which was obvious at the priority date. The PTFE coat when applied to a self-pulling corkscrew also had a non-obvious benefit of making a striking improvement in the extraction of the cork. The trial judge, Aldous J, held that the patent was invalid on the ground of obviousness because it was obvious to select the features of the claim for the first purpose notwithstanding that it was not obvious for the other purpose: [1989] RPC 307, 326-327. The Court of Appeal agreed with the judge, holding (pp 215-216) that it was self-evident that a PTFE coating would improve the penetration by any corkscrew and that the “golden bonus” or added benefit of the dramatic improvement in extraction of the cork would not found a valid patent as the claimed innovation was obvious for another purpose. Mr Waugh does not challenge this principle but submits that the 181 patent does not involve such an added benefit.’
813. Kobzeff is a US patent application from DSM which claims priority from the same document as EP155 and is substantially similar in its disclosure to EP155. Kobzeff proposes use of a protease for enzymatic lysis, optionally with a surfactant, from microbial cells including thraustochytrids. It gives conditions for the enzymatic treatment that include “pH conditions of approximately 5-9” ([0011], end of [0014] and claim 39 of Kobzeff).
814. DSM produced a comparison showing the differences between Kobzeff and EP155. DSM submitted the differences are not significant for the purposes of this case, but I have preferred simply to look at Kobzeff.
815. In what follows I have included all the parts of the specification to which the parties drew my attention, but added a number of my own.
816. Kobzeff is entitled ‘High quality lipids and methods for producing by enzymatic liberation from biomass’. Under ‘Background of the Invention’ the problems with existing methods of extracting lipids from biomass are said to include poor product quality due to chemically aggressive conditions of high temperature and high pH, high costs due to the need to dry the biomass and for additional equipment such as homogenisers and pressure vessels.
817. [0005] refers to the ‘fishy’ and ‘painty’ flavours of PUFAs which are primarily due to oxidation of the double bonds in the fatty acids and [0006] says the oxidative state of the lipid is strongly impacted by the processing conditions used to make the material.
818. Under ‘Summary of the Invention’ in [0007]-[0011], there are various statements as to the advantageous features of the invention.
‘[0011] A further method of the present invention is a method for liberating a lipid from a biomass comprising liberating the lipid at a temperature of about 10 C to about 80 C at a pH level of from about pH 5 to about pH 9. This method is conducted in the substantial absence of an extraction solvent.’
819. Mr Dueppen and Dr Kyle agreed that the reference to the substantial absence of “extraction solvent” is a reference to a solvent which solubilises the oil, like hexane, and not to the sort of solvent used in the FRIOLEX process like isopropanol.
820. In the Detailed Description, [0015] draws attention to the lipid being incorporated into an emulsion upon treatment with a protease (or protease + surfactant). [0016] indicates that the protease itself can help with breaking an emulsion by breaking down emulsion-stabilising proteins - a suggestion that Mr Dueppen trusted to be correct (Dueppen XX [T2/253/4-7]).
821. At [0024]-[0031] a preferred embodiment is set out, addressing the question of the emulsion. I set these paragraphs out with the indentation as in the specification:
‘[0024] One preferred embodiment of the process of the present invention includes:
[0025] Obtaining lipid-bearing single cell organisms
[0026] Treating with protease or a combination of surfactant and protease
[0027] Separating the lipid from the broth (may be an emulsion)
[0028] May require additional treatment with a polar organic solvent, salt, precipitating agent, another enzyme (protease or other kind), heating, cooling.
[0029] If the lipid from the above step is in the form of an emulsion, this product can be used “as is” or dried and used or treated to release the lipid from the emulsion
[0030] Treatment can include treatment with a polar organic solvent, salt, precipitating agent, another enzyme (protease or other kind), heating, cooling, etc.
[0031] The lipid can then be dried, refined, bleached, deodorized and/or reacted as needed.’
822. DSM suggested that this passage would put the skilled person in mind of at least 8 different treatments that could be used to treat the emulsion, plus combinations (i.e. 6 treatments expressly listed in [0030] plus centrifugation and gentle stirring coming within the “etc”).
823. However, DSM’s position was that the only treatment demonstrated to work in Kobzeff is the polar organic solvent (isopropanol) in Example 3. Their argument was that the use of that treatment, and not any of the others, would of course be consistent with the CGK as to what would and would not work to break a microbial oil emulsion.
824. Mara noted that treatments suggested in [0028] (and repeated in [0030]) for breaking the emulsion include salt and heating, both of which were part of the CGK for this purpose (see the EP801 CGK section above).
825. Next, Mr Dueppen was cross-examined on the first part of [0036] where it says:
‘In some cases, after the lipids are liberated from the biomass, the lipids can be separated directly from the undesired materials (e.g., cellular debris), such as by centrifugation, or other appropriate methods. In other cases, an agent such as an alcohol or other polar organic solvent can be added to facilitate the separation of the liberated lipid from the other material. In still other cases, a solvent can be added that will dissolve the lipid and facilitate the separation of the liberated lipid from the other material, e.g., by solvent extraction.’
826. In the remainder of the paragraph, reference is made to other patent documents which describe techniques for separating the lipids from undesired materials and combinations of lysis methods e.g. use of an enzyme with homogenisation.
827. Mr Dueppen agreed that [0036] discloses three options (Dueppen XX [T2/270/9 - 271/2]):
i) separation of the lipids directly with no solvent of any sort involved;
ii) use of a polar organic solvent, in a FRIOLEX-type process; or
iii) a solvent can be used to solubilise the oil, e.g. hexane, in which case no emulsion would arise.
828. Finally, the Examples of Kobzeff are the same as in EP155. I described those Examples at [238]-[246] above.
829. Mara acknowledged that Kobzeff does not disclose specifically raising the pH to demulsify the cell composition, but Mara contend that DSM’s reading of the claim for infringement covers raising the pH for other purposes (i.e. to be appropriate for the protease that is disclosed in EP155/Kobzeff). Mara contended that all the elements of the claim are disclosed on the following basis.
830. First, DSM accepted that the preamble and step (a) are disclosed in Kobzeff.
831. As to step (b), Mara contended it was disclosed for the following reasons. [0026] teaches use of a protease, and Kobzeff teaches at [0014] what pH to use for the enzymatic treatment: “pH conditions of approximately 5-9” (see also [0011]). Mara say that is a disclosure of a pH 5, of pH 9 and anywhere in between (as Mr Dueppen agreed in XX [T2/251/13-17]). Raising the pH, in particular to 8 or above, implicitly requires adding a base (Dueppen XX [T2/290/2-11]). Mara contended that it is implicit that the pH would be maintained throughout lysis, by addition of base (Dueppen XX [T3/287/12 - 288/8, 330/20-24]). Mara said it makes no difference to the claim that Kobzeff does not disclose having a pH of 8 or above for the purpose of demulsification - DSM’s infringement case is that that simply being at pH 8 or above during demulsification satisfies the claim.
832. Step (c) is also disclosed, in the form of any or all of steps (c1) (salt), (c2) (heating) and (c3) (agitation). Salt and heating are explicitly disclosed in [0030] as means of demulsification. Agitation is also implicitly disclosed, since it was essential to continuously stir vessels, and in any event would be necessary for proper mixing of base and/or salt, so the skilled person would always use stirring (Dueppen 1 ¶193; Dueppen XX [T2/264/23 - 265/6]).
833. Step (d) (separating the lipid) is also plainly disclosed, by centrifugation ([0015] and [0036]). It is implicit that, in order to do the RBD described in [0031], the lipid has been separated from the demulsified composition (Dueppen XX [T2/266/22 - 267/12]).
834. The lipid contains less than 5% by weight of an organic solvent (integer 1A.6). Although addition of an organic solvent is one option to assist with demulsification in [0030], it is certainly not mandatory; [0036] discloses not using any solvent as one approach (Dueppen XX [T2/270/9-17]). Salt and/or heat can be used instead to demulsify, and successful use of these would mean use of no solvent (Dueppen XX [T2/265/13 - 266/10]). So the process is disclosed without use of any organic solvent.
835. Alternatively, use of a polar organic solvent is disclosed as another route to breaking the emulsion. Treatment with an organic solvent is an option for breaking the emulsion in [0028] / [0030], as in the FRIOLEX process, and in the second sentence of [0036] as an alternative to using a completely solventless process (Dueppen XX [T2/270/9 - 271/2]). Having carried out a FRIOLEX-type process in this way, the skilled person would understand that the oil after separation would be substantially free of the solvent (Dueppen XX [T2/267/13-19]). Even if solvent of some sort had been used in the process, in the final product there would be no residual solvent left (Dueppen XX [T2/271/24 - 272/14]).
836. The lysis does comprise enzymatic treatment (integer 1A.7), namely the protease.
837. In Opening, DSM suggested that the teaching of a pH range of 5-9 and the teaching of addition of salt is not a disclosure of the combination of raising the pH to 8 or above and of adding a salt to demulsify.
838. In response, Mara made three points:
i) First, in the disclosure of the preferred embodiment, the addition of salt to demulsify is disclosed.
ii) Second, the process includes treating with protease or a combination of surfactant and protease, and the disclosure of the pH range of 5-9 in [0011] and [0014] for this purpose is a general disclosure that applies equally to [0026].
iii) Third, the process has to be done at some pH - it is not the disclosure of an option in the sense that there might be no pH at all. The teaching disclosing pH 5, pH 9 and everything in between is applicable to the process however carried out, as regards demulsification.
839. DSM also suggested that the only disclosure in Kobzeff of step (d) (separating a lipid) is the centrifugation in Example 3, which does not use the requisite pH.
840. Mara responded by saying that depended on an unrealistically restrictive reading of the document, which clearly discloses centrifugation to separate a demulsified lipid in [0015] and [0036], which is a general teaching in relation to separation. Mr Dueppen also agreed that there is implicit disclosure of separation in [0031] (see [833] above).
841. DSM contended the Kobzeff comes nowhere near clearly and unambiguously disclosing the specific combination of features of any of the claims of EP801. DSM suggested that in Mara’s Opening Skeleton, they had plucked various bits from the specification, left others behind, and compiled them into a process which Mara contends would be within the claim. DSM submitted that was not an anticipation case at all.
842. In addition, DSM contended that:
i) the case presented in Mara’s Opening Skeleton ¶310 does not include step (b) because it does not involve lysing the cells and then raising the pH of the lysed cell composition.
ii) the only disclosure of a process which includes step (d), separating a lipid, is Example 3. But in that example, the pH never gets above 7.3 and the separation process uses isopropanol, a polar organic solvent. The alternative to Example 3, suggested by Kobzeff at [0029], is to not separate the oil at all, but rather use the emulsion that is formed “as is”.
843. The teaching in Kobzeff of treating with a protease at a pH of about 5 to about 9 in [0011] is general teaching and generally applicable to the use of a protease or protease plus surfactant suggested by Kobzeff.
844. So far as the range of pH values is concerned, the issue is whether the disclosure can be treated as being of all values in the range. In other words, is this one of those instances where the implicit disclosure is that the skilled person may choose any value within the range - see Floyd LJ quoted in [699] above?
845. However, the Skilled Team would not have a free choice as to which pH to choose. In my view, the choice of pH would depend on the optimum conditions for the use of the enzyme in question - in this case Alcalase 2.4L FG.
846. There were various data sheets in evidence for Alcalase 2.4L FG from different manufacturers. Mr Speck KC cross-examined Mr Dueppen on one of them which specified the optimum conditions were pH 7-9, and Mr Dueppen agreed that all of the pHs between 7 and 9 were obvious.
847. Following that lysis step, Kobzeff’s preferred embodiment acknowledges that the lipid may need to be extracted from an emulsion, meaning that the emulsion must be broken - at least to some extent. Again, Kobzeff’s preferred embodiment proposes one or a combination of CGK methods. Although DSM contended in closing that the Skilled Team knows that the other methods do not work to break an emulsion, that misses the point.
848. Inevitably Kobzeff discloses separating the lipid from the demulsified cell composition and the final two integers of claim 1A are plainly present.
849. DSM are wrong that the only disclosure in Kobzeff of step (d) is Example 3. The Skilled Team reads Kobzeff with interest and is open to the idea that a prior art document may be teaching them something new. DSM’s point appeared to amount to a contention that the Skilled Team would not believe anything which was either (a) not CGK or (b) proved by an explicit example. However, there was no expert evidence to substantiate such an approach.
850. The upshot of all these points is that DSM escape anticipation of EP801 by Kobzeff because, in my view, Kobzeff does not plant the flag so far as the pH of 8 or above is concerned. The other elements of the claim are, in my view, disclosed in Kobzeff.
851. On my interpretation of the disputed parts of claim 1A, the obviousness analysis is straightforward. I agree that the use of a pH or 8 or above was obvious - see [846] above and [857] below. However, in case I am wrong on any of the construction issues or on points on Kobzeff’s disclosure, I propose to deal with the other arguments advanced.
852. In the alternative, Mara submitted that each of the independently valid claims of EP801 was obvious over Kobzeff.
853. Mara pointed out in Opening that DSM is constrained in light of Kobzeff’s close relationship to EP155 to accept that Kobzeff makes it obvious to use proteases to lyse Schizochytrium cells (with or without surfactants), and that the skilled person would be able to identify suitable proteases and conditions for their use (Dueppen 2 ¶117; Dueppen XX [T2/259/13 - 261/16, 284/17 - 285/22]).
854. On that basis, Mara submitted that the rest of claim 1 then follows without invention, as (they said) Mr Dueppen’s evidence confirmed.
855. First, Mara refer to the preferred embodiment in Kobzeff in [0024]-[0031], set out above.
856. In terms of the protease, Kobzeff suggests a pH range up to pH 9 (i.e. an alkaline range). The alkaline protease Alcalase is exemplified, and is plainly the enzyme to go to, according to Mr Dueppen (Dueppen XX [T2/285/4-9]).
857. Alcalase’s optimal pH range is 7 to 9 [D2/15/159]. Mr Dueppen agreed that any pH from 7 to 9 is obvious, confirmed that pH 9 itself is obvious, and the middle of the range (pH 8) is about as obvious as you could get (Dueppen XX [T2/285/23 - 287/8, T3/336/23-24]).
858. Since lysis - which may take 8 hours or more - will have an acidifying effect as acidic cell contents are released, the obvious thing to do is add base as lysis continues, to maintain the pH at the optimum level for the enzyme, all the way until the end of lysis. The pH when lysis comes to completion will therefore still be the pH selected as the optimum for enzyme activity, i.e. say pH 8 or pH 9 or whatever the pH was raised to initially (Dueppen XX [T2/287/12 - 289/25, T3/336/25 - 337/7]).
859. On that basis, Mara submitted it is therefore obvious to have a pH of ‘8 or above’ (whether or not on DSM’s case that this means 7.5 or above) at the end of the enzymatic lysis. According to DSM’s infringement case, that is enough to satisfy step (b).
860. DSM’s case, and Mr Dueppen’s evidence, was that:
i) Step (b) of claim 1A does not have to cause demulsification on its own (DSM’s EP801 skeleton argument ¶40 [S/4/69]; Dueppen 2 ¶¶104-105).
ii) It is the state of being at pH 8 or above after lysis that matters, not the act of raising the pH after lysis to get there (Dueppen 1 ¶288). DSM accordingly characterised the inventive concept as involving ‘using a pH of the lysed cell composition of 8 or above’ (as opposed to raising the pH to that level) (¶5A(a) of Re-Amended SoC on Infringement [B1/6/33], and see ¶5A(b) as to what is therefore said to infringe).
861. This was explored with Mr Dueppen at [T2/290/2 - 294/17]. The only distinction he could draw between EP801 and something that “just happens to fall in the same pH range” was the mental element of understanding that pH 8 (or above) helped with demulsification - he said that if it was just an accident that one satisfied the claimed feature, then that did not involve deliberately having a pH of 8 (or above) for demulsification. As Mara submitted, that makes no difference to obviousness of the claim. Naturally enough, it was common ground that the claim has no requirement of intention (DSM’s EP801 skeleton argument ¶42).
862. The result is that it is obvious to do what that step (b) requires, so Mara submitted. If that brings a bonus effect, that does not save the patent from invalidity.
863. When it came to dealing with the emulsion, solventless extraction was of course a CGK interest in 2010 (Dueppen 1 ¶¶69, 76). [0016] of Kobzeff also indicates that the protease itself can help with breaking an emulsion by breaking down emulsion-stabilising proteins - a suggestion that Mr Dueppen said can be trusted to be correct (Dueppen XX [T2/253/4-7]). Therefore, so Mara submitted, use of an enzyme reduces the prospect of a ‘strong’ emulsion in two ways relative to mechanical means such as homogenisation: first it is much gentler, requiring only gentle stirring rather than extreme shear forces (a point which I have not accepted to have been proved to be CGK), and secondly the breakdown of emulsion-stabilising proteins tends to reduce an emulsion as well.
864. In light of the CGK and [0027]-[0030] of Kobzeff, Mr Dueppen agreed that it would be obvious to use salt and/or heat, without any polar organic solvent (Dueppen XX [T2/296/8 - 297/20]). This satisfied step (c) by means of (c1) and/or (c2).
865. Alternatively, it would also be obvious to adopt Kobzeff’s suggestion of using an organic polar solvent such as isopropanol to break the emulsion, in a FRIOLEX approach, in combination with stirring. The solvent would then go in the aqueous phase upon separation of the oil, leaving the oil free of solvent (Dueppen 2 ¶¶122-126; Dueppen XX [T2/298/11-25]). As Mara submitted, this is also within the claims (see the construction of ‘less than 5% of organic solvent’ above).
866. As to claim 6A, adding salt and heat in either order is obvious. There is nothing in EP801 that suggests that there is an additional technical effect of applying salt and heat (above the effect that each brings), or that there is an advantage in one order rather than another (Dueppen XX [T3/338/22 - 339/23]). Mr Dueppen said that the order might matter, and that it would have to be tested (i.e. it would be obvious to try both ways round to see if it made a difference) (Dueppen XX [T2/291/21 - 298/10, T3/339/18 - 340/6]).
867. As to the agitation of claim 7A, stirring was a CGK approach to help with breaking an emulsion and is within the meaning of the claimed ‘agitation’ according to [0139] of EP801. It would be obvious to stir not only to break the emulsion, but in any case in order to give good mixing. Mr Dueppen agreed that it would be routine for there to be agitation in the form of continual stirring, and claim 7A does not give the skilled person anything of value (Dueppen XX [T2/296/4-7, T3/340/10-20]).
868. For those reasons, Mara contended all the independently valid claims were obvious.
869. DSM suggested there were three fundamental reasons to reject Mara’s case of obviousness.
870. First, DSM suggested that Kobzeff does not disclose or make it obvious to raise the pH “of a lysed cell composition” to any value, let alone 8 or above, as required by step (b) of claim 1A. DSM submitted that none of the obviousness cases explored in cross-examination by Mara faced up to this problem:
Mara’s first point was that it would be obvious to use a pH appropriate to the enzyme used for lysis. In the case of Alcalase that would include pH 8. wa DSM’s point was that However, have rejected DSM’s construction argument which underpins this point
Mara’s second point was that it would be obvious to continue to add base during lysis so that when lysis ends, the pH will still be at the pH selected for that enzyme. DSM suggested this suffers from the same problem as the first point above. If the pH is already at pH 8 when a lysed cell composition is formed, then there is no “ . This point fails for the same reason.
Mara’s third point was that the enzyme could be added first, and then the pH adjusted, but DSM said this would only be within the claim if sufficient time were allowed for lysis to occur before the pH was raised to 8 or above. Otherwise, this would not involve “adding a base to a lysed cell composition” as required by the claim. DSM submitted there was no evidence to establish such a case. contended by Dr Kyle, or let alone delay sufficient to allow significant lysis to occur) Once again, DSM’s argument depends on an interpretation of the claim which I have rejected.
871. In my judgment, for the Skilled Team in any practical implementation of Kobzeff with Alcalase 2.4L FG, the pH would inevitably be raised to 8 or above at one or more stages in the process. In a practical process, the Skilled Team would think it was impractical and unnecessary continuously to attempt to keep the pH at their target pH (and they would be correct). They would know (or would find out if they did not know this already) that lysis would tend to acidify the composition, so the pH would drop as lysis continued. Base would have to be added in an attempt to keep the pH in optimal range for Alcalase. Even if the target pH was 8 or 7.5, in any practical process, the target would inevitably be overshot so that at least at one (or more) points in the process, the pH would be raised to 8 (or 7.5) or above, with more base being added to anticipate the effect of the further lysis which would occur.
872. As a result, DSM’s contention that Mara failed to advance a factual case that is capable of rendering claim 1A obvious over Kobzeff is wrong.
873. DSM’s second point was that using a polar organic solvent such as isopropanol, such as in Example 3 of Kobzeff, is not within claim 1A for the additional reason that the process of obtaining a lipid would involve more than “5% by weight of an organic solvent”. On my construction of this integer, this would fall within claim 1A.
874. DSM submitted that the dependent claims come in at this point in the analysis, specifically claim 6A. DSM contended that if they are wrong about both the first and second points, that does not lead to obviousness of claim 6A. DSM’s argument was that Kobzeff Example 3 works, and it would still work if the pH used was a bit higher. Why then would the skilled person consider adding salt? DSM contended that no explanation was given as to why the skilled person would consider adding salt in this scenario and none was put to Mr Dueppen. I disagree. In this scenario there was nothing inventive in adding salt, a known CGK method which would or might assist with demulsification.
875. DSM’s third point was that Mr Dueppen gave clear evidence explaining why it would not be obvious in the light of Kobzeff to move to a full solventless extraction process which did not use isopropanol, relying on this passage in his evidence:
‘226. ... Powell Gilbert has explained to me the importance of avoiding hindsight in the context of inventive step, and I must put myself back in the position of the Skilled Bioprocessing Engineer at the EP 801 Priority Date but without knowledge of EP 801 itself.
227. In this context it is important to note that Kobzeff is not about solving the emulsion issue which arises with solventless extraction at all. Instead, as I have explained above at paragraph 151, the focus is on the lysis step. In particular using protease enzymes to lyse Schizochytrium cells. Kobzeff teaches that enzymatic lysis avoids the inconvenience and expense of the traditional drying step, and the mild conditions can also help in obtaining a high-quality oil. As I explained above, the Skilled Bioprocessing Engineer would consider Example 3, where enzymatic lysis was combined with an adapted FRIOLEX method using isopropanol to produce a good quality oil, interesting and would want to take this forward to investigate how the yield compared to traditional hexane extraction.
228. Therefore, although Kobzeff managed to avoid using the most problematic organic solvent, hexane, it used isopropanol and so did not even attempt to implement a truly solventless process. I do not believe it would be obvious to the Skilled Bioprocessing Engineer how to do a solventless process in light of Kobzeff, or whether such a process could be made to work, as there is little guidance on how an emulsion could be broken other than a generic list of options at paragraph [0030], which does not even mention considering the pH. I therefore do not believe it would be obvious to arrive at the method of the claims of EP 801.’
876. DSM argued that Mr Dueppen stood by this evidence in cross-examination. While he fairly accepted that the idea of using salt is not inventive, he was clear that an experimental design would be required to test the different techniques mentioned in [0030]; and that it was not obvious which ones (if any, other than isopropanol) would work to break a microbial oil emulsion. See T2/265/7 - 266/3 and especially 297/14 - 298/10.
877. However, these arguments only work on DSM’s construction of the < 5% integer, which I have rejected. Development of a solventless method is not required.
878. For all these reasons, I find that claims 1A, 6A and 7A of EP801 were obvious over Kobzeff. I accept Mara’s submissions as recorded in [855]-[868] above.
879. I can deal relatively succinctly with Hendrik because Mara’s case of obviousness of EP801 over Hendrik has a number of similarities to its case of obviousness of EP155 over Bijl, not least because Hendrik is a PCT application which claims priority from Bijl and reproduces a substantial part of it.
880. DSM produced a comparison showing the relatively few differences between Bijl and Hendrik. Only two of any significance were identified.
i) First, Hendrik includes some teaching about using a salt to aid in breaking the emulsion.
ii) Second, Hendrik contains two additional examples.
881. On the first point, at p6 lines 26-31, in the context of “Separation of oil from cell debris” Hendrik says that a “separation inducer, or agent to aid separation … [that] may aid in the formation of separate oil and aqueous phases” may be added. The one example of such an agent is “alkali metal salts, e.g. NaCl”.
882. On the second, Bijl’s Examples 1 & 2 are renumbered as 2 & 4 respectively, but are otherwise identical. Hendrik adds new Examples 1 & 3. Hendrik’s Examples 1 & 2 form a pair in which the key difference is that NaCl was added in the former. DSM contended that this pair appear to show that the addition of NaCl led to a much worse result, but the centrifuging step was different which may well have affected the comparison:
i) In Example 1 the yield of oil was just 9%, with the “oil layer” comprising 70% water and 20% medium components (e.g. cell debris). A “layer” comprising such components can only have been an emulsion.
ii) In Example 2 the yield was said to be 95%, and the list of components claims that this time separated oil, not an emulsion, was recovered.
883. Examples 3 & 4 also form a pair, with the former including the addition of NaCl, but they do not provide any data on oil yield, water content or oil quantity, so the effect of NaCl cannot be assessed.
884. Hendrik (like Bijl) refers in passing to “a pH shift (towards alkaline)” in the context of separation of microbial oil from cell wall debris. However, neither Mr Dueppen nor Dr Kyle suggested that this would be accepted. Mara’s case did not appear to refer to this teaching.
885. With these slight differences, the disclosure of Hendrik was the same as Bijl, so I have in mind the whole section set out above at [178]-[264] as to the disclosure of Bijl.
886. Although the arguments were not set out by reference to Pozzoli, it is a good discipline to adopt that framework. There is no real difference to the Skilled Team as at 1 June 2010 to the earlier priority dates. I have set out above the few respects in which their CGK had changed by that date. An important point to note is that the Skilled Team still would not assume or know, in advance of testing, that an emulsion from enzymatic lysis would be easier to break than an emulsion from mechanical lysis.
887. It is easiest to identify the issues relating to the differences between the disclosure of Hendrik and claim 1A of EP801 by reference to the steps required for Mara’s obviousness argument. I can start with DSM’s contentions as to the steps which were required:
i) Choose a Schizochytrium organism such as ATCC 20888. DSM pointed out that:
a) this is not mentioned as one of the organisms listed on p3 lines 16-26 but also
b) the claims of EP801 do not require a Schizochytrium organism, but Mara needs this step as a building block to get to enzymatic lysis and pH of 8 or above, which are requirements of the claims.
ii) Decide to do enzymatic lysis. DSM’s argument was that this had never been done before in the context of microbial oils or Schizochytrium in any context.
iii) Decide upon Alcalase. Not a requirement of EP801 but a necessary precursor to step iv) below.
DSM submitted that steps ii) and iii) would involve researching and testing to find which enzyme worked. They are not simply decisions to be made reading Hendrik.
iv) Decide to do enzymatic lysis at pH 8 or above.
v) Decide to add the base, to raise the pH to 8 or above, after adding the enzyme.
vi) Decide to maintain that pH throughout the lysis period.
vii) Finally and in addition, DSM pointed out that the Skilled Team would still have to decide how to break the emulsion following lysis.
DSM made the same argument here as they did for Bijl namely that the natural starting point would be to test out the examples. On that point, DSM submitted that either they work (unlikely, but in that case the last thing the skilled person would do is mess about with untested methods of lysis) or they don’t work (in which case why is the skilled person diverted to investigating new methods of lysis instead of solving the emulsion problem or abandoning Hendrik?). But if the skilled person is not starting with the examples, DSM submitted that Mara’s case appeared to be that the skilled person embarks on a research project armed only with the CGK methods that they know do not work. DSM submitted that how the skilled person gets within all of claims 1A, 6A and 7A is a mystery.
888. I reject Mara’s case of obviousness of EP801 over Hendrik, for two key reasons:
i) First, because the disclosure in Hendrik does not give the Skilled Team sufficient motivation to start to investigate using enzymatic lysis.
ii) Second, because it was not CGK that enzymatic lysis would create a weaker emulsion. Whilst I have no doubt that if the Skilled Team had experimented with enzymatic lysis, they would have found it resulted in an emulsion which was easier to break than emulsions formed from mechanical lysis, there was no evidence to support the notion that the Skilled Team would know or assume this in the absence of experimentation.
889. On that second point, Mara suggested during cross-examination that the use of an enzyme would create a weaker emulsion. That was rejected by Mr Dueppen. This is the same point that I discussed regarding Bijl and EP155 - see [281.iv)]-[284] above. As DSM submitted, the point has been constructed with hindsight.
890. Standing back, it can be seen that a major difference between Kobzeff and Hendrik is the fact that the former explicitly teaches enzymatic lysis and a pH range, whereas Hendrik does not call out enzymatic lysis, merely mentioning it as part of the list of possible lysis techniques but it did not feature in any further teaching and did not feature in any of the examples. A further difference is the teaching in Kobzeff [0016] that the protease itself can help with breaking an emulsion by breaking down emulsion-stabilising proteins, of which there is no trace in Hendrik. Hence, it is no surprise that, whilst the case of obviousness based on Kobzeff succeeds, the case on Hendrik does not.
891. On my decisions on construction and validity, no Gillette or Formstein issues arise. Indeed, as I understand matters, the possibility only arose if DSM’s order of steps argument succeeded. In the light of what I have said above, it is clear that DSM’s order of steps argument was the product of lawyers and divorced from the approach of the Skilled Team. In these circumstances, I do not propose further to lengthen this judgment with any discussion of the interesting issues which might have arisen if DSM’s argument had succeeded. Those are best tackled in a case where they need to be decided.
Overall Disposal
892. For the reasons explained above, I find:
i) EP155 was valid, although now expired. I understand infringement was admitted.
ii) EP740 invalid on several bases namely, (1) the proposed amendments were not permissible as not being clear and concise and because they would have added matter; (2) even if the proposed amendments had been allowed, claim 1B would have been invalid for obviousness over Fabritius; and (3) whether amended or not, EP740 was plainly invalid for breadth of claim insufficiency.
iii) EP801 invalid for obviousness over Kobzeff.
893. I ask the parties to arrange a form of order hearing within a reasonable period following the hand down of this judgment and in any event before the end of the Easter term. Pending that hearing, I will extend time for the filing of any Appeal or application to the Court of Appeal for permission to appeal until 21 days after judgment on that hearing.




















