|
|
Notice of the making of this Statutory Instrument was published in
|
|
|
"Iris Oifigiúil" of 3rd September, 2010.
|
|
|
I, MARY HARNEY, Minister for Health and Children, in exercise of the powers conferred on me by section 3 of the European Communities Act 1972 (No. 27 of 1972) and for the purpose of giving effect to Commission Directive 2010/4/EU 1 of 8 February 2010, hereby make the following regulations:
|
|
|
Citation
|
|
|
1. These Regulations may be cited as the European Communities (Cosmetic Products) (Amendment) (No. 2) Regulations 2010.
|
|
|
Collective citation
|
|
|
2. The collective citation "the European Communities (Cosmetic Products) Regulations 2004 to 2010" includes these Regulations.
|
|
|
Commencement
|
|
|
3. (1) Subject to paragraph (2), these Regulations come into operation on1 December 2010.
|
|
|
(2) Column f (requirements relating to labelling) of Reference Number 208 (inserted by Regulation 6(1) of these Regulations) in Part 1 of Schedule 3 to the Principal Regulations comes into operation-
|
|
|
(a) on 1 November 2011, so far as regards its application to the placing on the market of a cosmetic product which-
|
|
|
(i) contains a substance mentioned in Column b of the reference number, and
|
|
|
(ii) is manufactured or imported on or after that date,
|
|
|
and
|
|
|
(b) on 1 November 2012, so far as regards its application to the placing on the market of any other cosmetic product which contains a substance so mentioned.
|
|
|
Interpretation
|
|
|
4. In these Regulations, "Principal Regulations" means the European Communities (Cosmetic Products) Regulations 2004 ( S.I. No. 870 of 2004 ), as amended.
|
|
|
Amendment of Regulation 4(1) of Principal Regulations
|
|
|
5. The definition of "Directive" in Regulation 4(1) of the Principal Regulations is further amended by the addition to it of a reference to Commission Directive 2010/4/EU.
|
|
|
Amendment of Schedule 3 to Principal Regulations
|
|
|
6. Subject to Regulation 3(2), Schedule 3 to the Principal Regulations is amended-
|
|
|
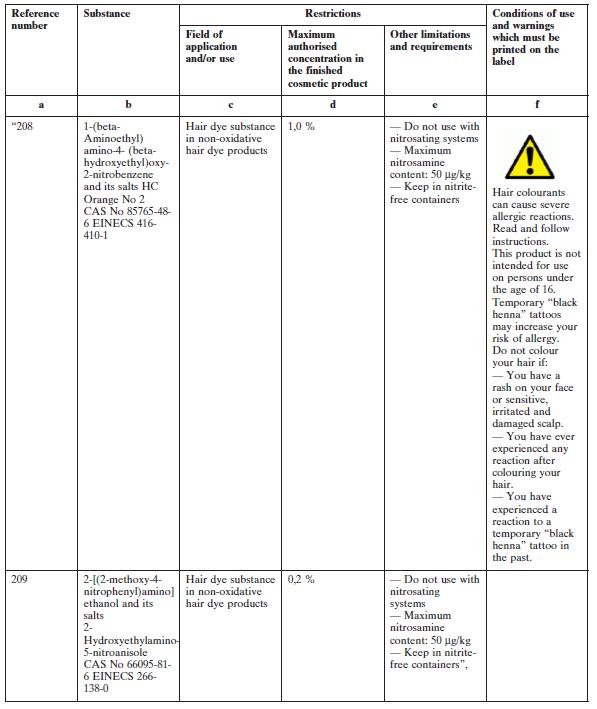
(1) in Part 1, by the insertion, after Reference Number 207, of the following reference numbers:
|
|
|
|
|
|
and
|
|
|
(2) in Part 2, by the deletion of Reference Numbers 26 and 29.
|
|
|

|
|
|
Given under my Official Seal,
|
|
|
1 September 2010.
|
|
|
MARY HARNEY,
|
|
|
Minister for Health and Children.
|
|
|
EXPLANATORY NOTE
|
|
|
(This note is not part of the Instrument and does not purport to be a legal interpretation.)
|
|
|
These Regulations give effect to Commission Directive 2010/4/EU of 8 February 2010. They amend the European Communities (Cosmetic Products) Regulations 2004 to 2010 and provide for restrictions in relation to the use, storage and labelling of certain hair dye products.
|
|
|
1 OJ L36, 9.2.2010, p.21
|


