Trinity
Term
[2017] UKSC 48
On appeals from: [2015] EWCA Civ 555 and [2015] EWCA Civ 666
JUDGMENT
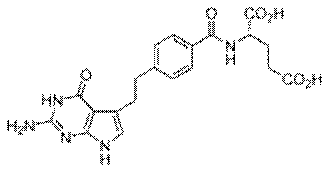
Actavis UK Limited and others (Appellants) v
Eli Lilly and Company (Respondent)
Eli Lilly and Company (Appellant) v Actavis UK Limited and others
(Respondents)
Eli Lilly and Company (Appellant) v Actavis UK Limited and others
(Respondents)
before
Lord Neuberger, President
Lord Mance
Lord Clarke
Lord Sumption
Lord Hodge
JUDGMENT GIVEN ON
12 July 2017
Heard on 4, 5 and 6 April
2017
|
Actavis and
others
Daniel Alexander QC
Thomas Raphael QC
Isabel Jamal
(Instructed by Bird
& Bird LLP)
|
|
Eli Lilly and
Company
Thomas Mitcheson QC
Andrew Waugh QC
Stuart Baran
(Instructed by
Hogan Lovells International LLP)
|
LORD NEUBERGER: (with whom
Lord Mance, Lord Clarke, Lord Sumption and Lord Hodge agree)
1.
The issue raised on this appeal and cross-appeal is whether three
products manufactured by the Actavis group of companies (“Actavis”) would infringe
a patent whose proprietor is Eli Lilly & Company (“Lilly”), namely European
Patent (UK) No 1 313 508 (“the Patent”), and its corresponding designations in
France, Italy and Spain.
2.
This judgment was circulated in draft to the parties’ legal representatives
in the normal way on 5 July 2017, on the basis that it would be handed down a
week later. On the following day, just after midday, Actavis’s solicitors
emailed the Court expressing concern about the potential prejudice which their
clients could suffer if they did not know of the outcome of this appeal until
12 July. Not least because publication of our decision could have an effect on
the share prices of Actavis or Lilly or both of them, the Court proposed to the
parties’ respective solicitors that we should announce our decision at once,
while maintaining the intention, in accordance with this Court’s usual
practice, to hand down the judgment a week after circulation of the draft. This
was agreed by both solicitors, and accordingly on 7 July at 11.30 am, the
following announcement appeared on the Court’s website:
“The Supreme Court allows Eli
Lilly’s appeal and holds that Actavis’s products directly infringe Eli Lilly’s
patent in the United Kingdom, France, Italy and Spain. The Court dismisses
Actavis’s cross-appeal on the basis that if its products did not directly
infringe, they would indirectly infringe to the extent held by the Court of
Appeal.”
Accordingly, these are technically the reasons for those
conclusions.
The factual and
technical background
The factual background
3.
Pemetrexed is a chemical which has been known for some time to have
therapeutic effects on cancerous tumours. However, when used for that purpose
on its own, pemetrexed can often have seriously damaging, sometimes even fatal,
side-effects. Accordingly, its use as an anti-cancer drug was effectively
precluded in practice. The essential disclosure of the Patent was that the
damaging side-effects could largely be avoided if a compound called pemetrexed
disodium was administered together with vitamin B12. This has enabled
pemetrexed disodium to be used for treatment in the form of a medicament which
includes the vitamin. Such a medicament has been successfully marketed, under
the brand name Alimta, by Lilly since 2004.
4.
The Patent primarily claims the use of pemetrexed disodium in the
manufacture of a medicament for use in combination with vitamin B12 (and,
optionally, folic acid) for the treatment of cancer.
5.
Pemetrexed itself is a member of a class of chemicals known as
antifolates, and its molecular structure is shown below, with C, N, O and H being
respectively the chemical symbols for carbon, nitrogen, oxygen and hydrogen;
and the unallocated points on the chains and the rings being carbon.
6.
The presence of the two -CO2H units results in pemetrexed
being an acid (hence it is also known as pemetrexed diacid), or as it is
sometimes called, a free acid. When pemetrexed is dissolved in water, the
hydrogens in those two units separate from the rest of the molecule as
positively charged entities, protons, and the rest of the molecule becomes a
negatively charged entity called an anion. The structure of pemetrexed disodium
is similar except that, instead of the two -CO2H units, it has two
-CO2Na units (Na being the symbol for sodium). Pemetrexed disodium
dissolves in water, where the two sodiums separate from the rest of the
molecule as positively charged entities called cations, and the rest of the
molecule becomes an anion. Because it is the pemetrexed anion which is of
interest, the sodium cation is often referred to as a counter-ion. A substance
such as pemetrexed disodium, where the acidic hydrogens have been replaced, is
known chemically as a salt.
7.
Although one might have thought that the actual invention should have
been characterised as a disclosure that pemetrexed could be administered safely
if it was combined in a medicament with vitamin B12, the claimed invention in
the Patent is, as mentioned in para 4 above, the manufacture of such a
medicament. This formulation was required by the then-prevailing law contained in
article 52(4) of the European Patent Convention 1973 (“EPC 1973”), which
prohibited from patentability any method of treatment of humans or animals.
This led to inventions which otherwise might have been expected to be expressed
as being new therapeutic treatments being cast as manufacturing claims. Such
claims are known as Swiss form claims, and they were illuminatingly discussed
by Kitchin J in Ranbaxy (UK) Ltd v Astrazeneca AB [2011] FSR 45, paras
42 to 60. As he explained, the prohibition was substantially modified in article
53 in the European Patent Convention 2000 (“EPC 2000”), but that modification
had not come into force when Lilly applied for the Patent.
8.
Actavis’s proposed products involve pemetrexed compounds being used
together with vitamin B12 for cancer treatment. However, rather than pemetrexed
disodium, the active ingredient in those products (“the Actavis products”) is
(a) pemetrexed diacid, (b) pemetrexed ditromethamine, or (c) pemetrexed
dipotassium. In other words, rather than including the disodium salt referred
to in claim 1 of the Patent, the Actavis products include as the active
ingredient (a) pemetrexed itself (ie the free acid), or pemetrexed with the
hydrogens on the two -CO2H units replaced by (b) tromethamine, or
(c) potassium. Actavis contend that, because they intend to use the Actavis
products which do not include pemetrexed disodium, the claims of the Patent,
which are expressed as involving the use of pemetrexed disodium, would not be
infringed. By contrast, Lilly contends that there would be either direct or
indirect infringement of the Patent if Actavis launch any of the Actavis
products on the market in the UK or in France, Italy, or Spain. The allegation
of direct infringement is based simply on the proposition that marketing or use
of the Actavis products would infringe the Patent; indirect infringement is
said to arise because pemetrexed disodium is claimed to be involved in the
preparation of the Actavis products before they are administered.
9.
After a four-day trial, Arnold J decided that none of the Actavis
products would directly or indirectly infringe the Patent in the UK or in
France, Italy or Spain - [2015] Bus LR 154; [2015] RPC 6. The Court of Appeal
allowed Lilly’s appeal to the limited extent of holding that there would be
indirect infringement in the four jurisdictions, but they agreed with the Judge
that there would be no direct infringement - [2015] Bus LR 1068. Lilly appeals
against the rejection of its case that there would be direct infringement, and
Actavis cross-appeal against the rejection of their case that there would be no
indirect infringement.
10.
As Floyd LJ explained in the Court of Appeal, the appeal raises the
issue of the correct approach under UK law (and the law of the three other
states) to the interpretation of patent claims, and in particular the
requirement of EPC 2000 to take account of “equivalents”, and also the extent
to which it is permissible to make use of the prosecution history of a patent
when determining its scope. The issue on the cross-appeal is rather more
fact-specific, namely whether the application of the law of contributory
infringement justifies a finding of indirect infringement in this case.
11.
It is appropriate to start by setting out the relevant provisions of the
Patent and the knowledge of its assumed addressee, topics on which my account
is largely taken from the clear judgment of Floyd LJ in the Court of Appeal. I
will then turn to the issue of direct infringement, which involves considering
the proper approach to that issue generally, and also the relevance of the
prosecution history. I will then consider the position in the three other
states and finally I will address the issue of indirect infringement.
The specification and claims in the Patent
12.
The Patent is entitled “Combination containing an antifolate and
methylmalonic acid lowering agent”, and it has a claimed priority date of 30
June 2000.
13.
The specification begins at para [0001] by stating that “[p]otentially,
life-threatening toxicity remains a major limitation to the optimal
administration of antifolates”. It then explains at para [0002] that
antifolates work by inhibiting anti-folate-requiring enzymes by competing with
reduced folates for binding sites on those enzymes. The specification
identifies several antifolate drugs as being in development, including Lilly’s
branded product Alimta.
14.
The specification then explains at para [0003] that a limitation to the
development of these drugs is that they may be associated with substantial
toxicity, including mortality, for some patients. These toxicity effects had
led to the abandonment of the development of some antifolates. In para [0004]
the specification explains that previous work had been done on the use of folic
acid as a treatment for toxicity in this area. It also records work on vitamin
B12 as a predictor of cytotoxic events.
15.
The specification then states in para [0005]:
“Surprisingly and unexpectedly, we
have now discovered that certain toxic effects such as mortality and
non-hematologic events, such as skin rashes and fatigue, caused by antifolates,
as a class, can be significantly reduced by the presence of a methylmalonic
acid lowering agent as vitamin B12, without adverse adversely affecting
therapeutic efficacy. The present invention thus generally relates to a use in
the manufacture of a medicament for improving the therapeutic utility of
antifolate drugs by administering to the host undergoing treatment with a
methylmalonic acid lowering agent as vitamin B12.”
16.
Para [0006] of the specification continues:
“Additionally, we have discovered
that the combination of a methylmalonic acid lowering agent as vitamin B12 and
folic acid synergistically reduces the toxic events associated with the
administration of antifolate drugs. Although, the treatment and prevention of
cardiovascular disease with folic acid in combination with vitamin B12 is
known, the use of the combination for the treatment of toxicity associated with
the administration of antifolate drugs was unknown heretofore.”
17.
These early, general statements are made in relation to antifolates as a
class. However, at para [0010] the specification says, in what is known as a
consistory clause, that the invention:
“specifically provides the use of
the antifolate pemetrexed disodium in the manufacture of a medicament for use
in combination therapy for inhibiting tumour growth in mammals wherein said
medicament is to be administered in combination with a methylmalonic acid
lowering agent selected from vitamin B12 and pharmaceutical derivatives
thereof.”
18.
Having referred specifically to pemetrexed disodium, the specification
reverts to generality at para [0016], where it states:
“The current invention concerns
the discovery that administration of a methylmalonic acid lowering agent such
as vitamin B12 or a pharmaceutical derivative thereof, in combination with an
antifolate drug such as pemetrexed disodium reduces the toxicity of the said
antifolate drug.”
19.
Para [0022] contains a definition:
“The terms ‘antifolate’ and
‘antifolate drug’ generally refer to a chemical compound which inhibits at
least one key folate-requiring enzyme of the thymidine or purine biosynthetic
pathways ... by competing with reduced folates for binding sites of these
enzymes. The ‘antifolate’ or ‘antifolate drug’ for use in this invention is
Pemetrexed Disodium (ALIMTA®), as manufactured by Eli Lilly & Co.”
20.
The invention is then illustrated by reference to a number of examples
relating to animal and human tests, in which the only antifolate used is
pemetrexed disodium. At para [0035] the specification states that animals were
treated with “pemetrexed disodium (ALIMTA®) (100 mg/kg or 150 mg/kg)
once daily … by intraperitoneal injection alone or along with folic acid”. The
specification also indicates at para [0044] that, in a typical clinical
evaluation using cancer patients, the antifolate is to be administered in four
doses over a two-week period by rapid intravenous injection.
21.
Turning to the claims, it is only necessary for present purposes to
refer to claims 1 and 12, which are in these terms:
“1. Use of pemetrexed
disodium in the manufacture of a medicament for use in combination therapy for
inhibiting tumour growth in mammals wherein said medicament is to be
administered in combination with vitamin B12 or a pharmaceutical derivative
thereof [which it then specifies].”
“12. A product containing
pemetrexed disodium, vitamin B12 or a pharmaceutical derivative thereof said
pharmaceutical derivative [which it again specifies], and, optionally, a folic
binding protein binding agent selected from [a specified group of chemicals
including folic acid], as a combined preparation for the simultaneous, separate
or sequential use in inhibiting tumour growth.”
The notional addressee of the Patent
22.
A patent is interpreted on the basis that it is addressed to a person or
group of persons who is or are likely to have a practical interest in the
claimed invention, ie through the eyes of a person or persons skilled in the
art. There is now no challenge to the Judge’s conclusion that the notional
addressee of the Patent would be a group consisting of an oncologist and a
chemist, a conclusion upheld by the Court of Appeal.
23.
The Judge found that the common general knowledge of an oncologist as at
the relevant time, 2001/2002, included the following:
i) Antifolates were
used in cancer chemotherapy, but their use caused toxic side effects which it
would be desirable to avoid or reduce.
ii) Pemetrexed was the
subject of clinical trials for use in chemotherapy, and it targeted multiple
enzymes and was administered intravenously.
iii) The only form of
pemetrexed which had been shown to be effective and safe to any extent was
pemetrexed disodium, which was manufactured by Lilly under the trade mark
Alimta.
iv) The characteristics
of both vitamin B12 and folic acid were well understood, and it was well known
that there were many different safe and effective forms of both available.
24.
The Judge also concluded that oncologists did not think about drugs such
as pemetrexed in their ionic form, nor did they consider issues regarding the
choice of counter-ion or the effect, if any, of counter-ions on the efficacy,
safety or other properties of the drug. This was the province of the chemist
and, because the properties of different salt forms and free acids were
difficult to predict, a chemist would need to address any such issue by
conducting experiments.
25.
The Judge made the following findings as to the common general knowledge
of a chemist as at 2001/2002:
i) Where a drug is or
is based on an acid, different salts of the parent acid can be formed by
reacting it with a complementary base or acid. The salt will often have
different properties from the parent acid, and different salts will often have
different properties from each other. So, salt screening is a routine but
important exercise in determining the most suitable form of a drug.
ii) The facts set out
in paras 5 and 6 above.
iii) Solid salts consist
of the anions and cations regularly arranged in a fixed lattice structure.
Because the cations and anions are present in fixed proportions and in fixed
relative positions it is possible to speak meaningfully of the salt as being
present in solid form.
iv) When a salt is
dissolved in water, the ions dissociate, forming free cations and anions in
solution. Although the salt ceases to exist, it is common to refer to “a salt
solution” or “a salt in solution”.
v) The salt form can
have a significant impact on the effectiveness of a drug in that it can modify
many aspects of the drug.
vi) When considering a
drug for intravenous chemotherapy, the solubility of the salt form is crucial,
as good solubility is an indicator of how likely it is that the drug will be
absorbed in the gut.
vii) But if a salt is too
soluble, it cannot be made in solid form.
viii) In general, there
can be many dead-ends and false leads when attempting to prepare salts of a
parent molecule for the first time.
ix) One cannot predict
(a) whether one could make a particular salt form of a parent molecule, (b)
what its properties would be once it was made or (c) whether it would affect
the efficacy of the drug.
26.
The Judge made specific findings about a chemist’s state of knowledge
about three types of salts and about free acids:
i) Sodium was
generally the preferred counter-ion, and so would be first choice. Sodium salts
generally were not toxic, and would be expected to be reasonably soluble, but
they were not always easy to make.
ii) Potassium
salts were also generally soluble, but there were exceptions. There were
concerns about the potential toxicity of such salts, which was particularly
significant if large quantities of the drug were involved.
iii) There
were only a small handful of examples of tromethamine salts being used in 2001.
It was known that tromethamine salts might well be too soluble, so one would
not be able to make and harvest the solid form.
iv) In
principle, the acidic parent molecule could be administered in the form of the
free acid. But it was often necessary to change from the free acid to a salt
form for various reasons including solubility.
Direct infringement
27.
In a nutshell, the rival contentions are these. Lilly argues that the
Actavis products infringe the Patent because they are medicaments to be used as
a treatment for cancer consisting of pemetrexed diacid, or a pemetrexed salt,
with vitamin B12, which represents the essence of the teaching and claim of the
Patent. By contrast, Actavis argues that their products do not infringe because
the claims of the Patent are limited to a specific pemetrexed salt, namely
pemetrexed disodium, and the Actavis products contain either pemetrexed diacid
or different pemetrexed salts.
The legislative context
28.
The domestic provision governing direct patent infringement is section
60(1) of the Patents Act 1977. However, section 130(7) declares that certain
provisions of that Act, including section 60, are “so framed as to have, as
nearly as practicable, the same effects in the United Kingdom as the
corresponding provisions of the European Patent Convention … have in the
territories to which [that Convention applies]”. Accordingly, it is common
ground that it is appropriate to consider the present case by reference to the EPC
2000.
29.
Article 69(1) EPC 2000 provides that “[t]he extent of the protection
conferred by a European patent … shall be determined by the claims”, although
it is followed by another sentence, namely “[n]evertheless, the description and
drawings shall be used to interpret the claims”.
30.
As a matter of ordinary language, it is quite clear that the only type
of pemetrexed compound to which the Patent’s claims expressly extend is
pemetrexed disodium. One only needs to read claim 1 and claim 12 to justify
that: as a matter of ordinary language, “pemetrexed disodium” means that
particular salt, and no other salt, let alone the free acid. If the first few
words of each claim were not enough to make this good, the contrast between the
specific reference to pemetrexed disodium and the wider reference to “vitamin B12
or a pharmaceutical derivative thereof” underlines the point. As Floyd LJ said,
this conclusion is also supported by what is said in the specification - eg in
paras [0010] and [0022] quoted above. It is fair to say that para [0016] could
be said to point the other way, but it is far too weak a basis for even arguing
that the Patent’s claims extend, as a matter of language, to pemetrexed
compounds other than pemetrexed sodium.
31.
In these circumstances, The Protocol on the Interpretation of article 69
as amended in 2000 (“the Protocol”) is crucial to Lilly’s contention that the
scope of protection afforded by the Patent extends to the Actavis products. The
Protocol provides:
“Article 1
General principles
Article 69 should not be
interpreted as meaning that the extent of the protection conferred by a
European patent is to be understood as that defined by the strict, literal
meaning of the wording used in the claims, the description and drawings being
employed only for the purpose of resolving an ambiguity found in the claims.
Nor should it be taken to mean that the claims serve only as a guideline and
that the actual protection conferred may extend to what, from a consideration
of the description and drawings by a person skilled in the art, the patent
proprietor has contemplated. On the contrary, it is to be interpreted as
defining a position between these extremes which combines a fair protection for
the patent proprietor with a reasonable degree of legal certainty for third
parties.
Article 2
Equivalents
For the purpose of determining the
extent of protection conferred by a European patent, due account shall be taken
of any element which is equivalent to an element specified in the claims.”
The original Protocol was agreed in 1973; the amendments
made in 2000 effected very slight modifications to what is now article 1, and
introduced article 2 for the first time.
32.
The drafting of the Protocol bears all the hallmarks of the product of a
compromise agreement. This is unsurprising. There is an inevitable conflict
between the desirability of giving an inventor an appropriate degree of
protection in a particular case and the need for clarity of principle as to the
extent of such protection generally; and, of course, there is an unavoidable
tension between the appropriateness of giving an inventor a monopoly and the
public interest in maximising competition. In addition, the EPC 2000 and the
Protocol apply in many different states which have different traditions and
approaches in relation to the law of patents. In that connection, as the
Supreme Court observed in Schütz (UK) Ltd v Werit (UK) Ltd (Nos 1 to 3) [2013] Bus LR 565; [2013] RPC 16, para 40, “complete consistency of approach” between
different national courts of the EPC states “is not a feasible or realistic possibility
at the moment”, but nonetheless “it is sensible for national courts at least to
learn from each other and to seek to move towards, rather than away from, each
other’s approaches”.
33.
More specifically, two points appear to be clear from the Protocol. The
first, which can be deduced from article 1, is that the scope of protection
afforded to a patentee is not to be limited by the literal meaning of the
claims. However, it is not at all clear how far a court is permitted to move
away from the literal meaning. I do not consider that the last part of the
first sentence of article 1 only enables the description (ie the specification)
and the drawings to be taken into account when interpreting the claims, in
cases where the claims would otherwise be ambiguous. Any doubt about this must
be put to rest by the second and third sentences, which make it clear to my
mind that that would be too narrow a reading. However, it is very hard to be
confident how far they were intended to permit a court to go beyond the actual
language of a claim when interpreting a claim. Secondly, it is apparent from article
2 that there is at least potentially a difference between interpreting a claim
and the extent of the protection afforded by a claim, and, when considering the
extent of such protection, equivalents must be taken into account, but no
guidance is given as to precisely what constitutes an equivalent or how
equivalents are to be taken into account.
34.
The question of how far one can go outside the wording of a claim to
enable the patentee to enjoy protection against products or processes which are
not within the ambit of the actual language, construed in accordance with
ordinary principles of interpretation, has been considered in three significant
UK cases and in a number of significant cases decided in the courts of other
Convention states.
The domestic case law
35.
The UK case of Catnic Components Ltd v Hill & Smith Ltd [1982]
RPC 183 was decided under the previous, purely domestic, legislation, the
Patents Act 1949. At pp 242 to 243, Lord Diplock deprecated the notion that
there were two types of infringement, “textual infringement” and “infringement
of the ‘pith and marrow’ of the invention”, and said that there was “a single
cause of action”, which involved asking the question:
“whether persons with practical
knowledge and experience of the kind of work in which the invention was
intended to be used, would understand that strict compliance with a particular
descriptive word or phrase appearing in a claim was intended by the patentee to
be an essential requirement of the invention so that any variant would fall
outside the monopoly claimed, even though it could have no material effect upon
the way the invention worked.”
He continued:
“The question, of course, does not
arise where the variant would in fact have a material effect upon the way the
invention worked. Nor does it arise unless at the date of publication of the
specification it would be obvious to the informed reader that this was so.
Where it is not obvious, in the light of then-existing knowledge, the reader is
entitled to assume that the patentee thought at the time of the specification
that he had good reason for limiting his monopoly so strictly and had intended
to do so, even though subsequent work by him or others in the field of the
invention might show the limitation to have been unnecessary. It is to be
answered in the negative only when it would be apparent to any reader skilled
in the art that a particular descriptive word or phrase used in a claim cannot
have been intended by a patentee, who was also skilled in the art, to exclude
minor variants which, to the knowledge of both him and the readers to whom the
patent was addressed, could have no material effect upon the way in which the
invention worked.”
36.
In that case, the patent was for a novel type of galvanised steel
lintel, which the relevant claim described as including a rear support back
plate “extending vertically” from a horizontal plate. The allegedly infringing
article included a rear support member which was inclined between 6 degrees and
8 degrees from the vertical. Overruling the Court of Appeal’s decision that
this meant that there was no infringement, Lord Diplock said at p 244, that it
would have been:
“obvious to a builder familiar
with ordinary building operations that the description of a lintel in the form
of a weight-bearing box girder of which the back plate was referred to as
‘extending vertically’ from one of the two horizontal plates to join the other,
could not have been intended to exclude lintels in which the back plate
although not positioned at precisely 90 degree to both horizontal plates was
close enough to 90 degree to make no material difference to the way the lintel
worked when used in building operations.”
He then added this:
“No plausible reason has been
advanced why any rational patentee should want to place so narrow a limitation
on his invention. On the contrary, to do so would render his monopoly for
practical purposes worthless, since any imitator could avoid it and take all
the benefit of the invention by the simple expedient of positioning the back
plate a degree or two from the exact vertical.”
37.
A few years later, Hoffmann J (as he then was) gave judgment in Improver
Corpn v Remington Consumer Products Ltd [1990] FSR 181. The case concerned
a patent for a depilator, known as the “Epilady”, which worked by trapping
hairs in a rotating “coiled helical spring”, and the alleged infringement
worked in very much the same way save that, instead of a spring, it used a
slotted rubber rod. The case had already gone on an interlocutory issue to the
Court of Appeal, where it was held that Lord Diplock’s approach in Catnic [1982]
RPC 183 was consistent with the 1977 Act, the EPC 1973 and the Protocol as it
then was - see [1989] RPC 69.
38.
At [1990] FSR 181, 189, Hoffmann J suggested the following approach, largely
based on his reading of the reasoning in Catnic [1982] RPC 183, 242 to
243:
“If the issue was whether a
feature embodied in an alleged infringement which fell outside the primary,
literal or a contextual meaning of a descriptive word or phrase in the claim
(‘a variant’) was nevertheless within its language as properly interpreted, the
court should ask itself the following three questions:
(1) Does the variant have a
material effect upon the way the invention works? If yes, the variant is
outside the claim. If no -
(2) Would this (ie that the
variant had no material effect) have been obvious at the date of publication of
the patent to a reader skilled in the art? If no, the variant is outside the
claim. If yes -
(3) Would the reader
skilled in the art nevertheless have understood from the language of the claim
that the patentee intended that strict compliance with the primary meaning was
an essential requirement of the invention? If yes, the variant is outside the
claim.
On the other hand, a negative
answer to the last question would lead to the conclusion that the patentee was
intending the word or phrase to have not a literal, but a figurative meaning
(the figure being a form of synecdoche or metonymy) denoting a class of things
which included the variant and the literal meaning, the latter being perhaps
the most perfect, best-known or striking example of the class.”
39.
Hoffmann J then proceeded to apply those three questions to the facts of
the case before him. He held that the first two questions were to be answered
in the patentee’s favour and then turned to the third question. On that
question, he held that the patentee failed for the reasons he gave at p 197,
namely that “[t]he rubber rod is not an approximation to a helical spring”,
that “the spring [cannot] be regarded as an ‘inessential’ or the change from
metal spring to rubber rod as a minor variant”, and that it could be
appreciated that the patentee would wish to restrict his claim to helical
springs as “[i]t would be obvious that the rubber had problems of hysteresis
which might be very difficult to overcome”.
40.
Thereafter, for the next 15 years or so, this three-stage approach was
almost routinely applied by judges in UK patent infringement cases, where the
three “Improver questions” were subsequently renamed the three “Protocol
questions” - see Wheatley v Drillsafe Ltd [2001] RPC 7, para 23.
41.
Lord Hoffmann (as he had by then become) addressed the issue again in
his speech in Kirin-Amgen Inc v Hoechst Marion Roussel Ltd [2005] RPC 9,
where one of the issues was whether a protein manufactured by gene-activation
infringed a patent relating to production of the same protein by recombinant
DNA technology. At paras 27 to 35, Lord Hoffmann discussed “the English rules
of construction”. At paras 30 to 32 he effectively equated Lord Diplock’s
approach to patents in Catnic [1982] RPC 183, 243 with “purposive
construction” of commercial contracts. At para 34, he said that “[t]he question
is always what the person skilled in the art would have understood the patentee
to be using the language of the claim to mean. And for this purpose, the
language he has chosen is usually of critical importance”.
42.
Lord Hoffmann then turned to the doctrine of equivalents, which he
explained in para 37 had been developed in the United States courts and
“allow[ed] the patentee to extend his monopoly beyond his claims”, so as to
prevent “the unscrupulous copyist [from making] unimportant and insubstantial
changes and substitutions in the patent which, though adding nothing, would be
enough to take the copied matter outside the claim, and hence outside the reach
of law”, quoting Jackson J in Graver Tank & Manufacturing Co Inc v Linde
Air Products Co 339 US 605, 607 (1950). Lord Hoffmann expressed concern
that “once the monopoly had been allowed to escape from the terms of the
claims, it is not easy to know where its limits should be drawn”, and concluded
that, rather than adhering to literalism and adopting the doctrine, the
solution was “to adopt a principle of construction which actually gave effect
to what the person skilled in the art would have understood the patentee to be
claiming”, as Lord Diplock had done in Catnic [1982] RPC 183. He also
said that article 69 EPC 2000 “firmly shuts the door on any doctrine which
extends protection outside the claims” (see at paras 39 and 42 to 44).
43.
Having considered the issue in the three preceding paragraphs of his
speech, at para 48 Lord Hoffmann stated that the approach adopted by Lord
Diplock was “precisely in accordance with the Protocol”, as it was “intended to
give the patentee the full extent, but no more than the full extent, of the
monopoly which a reasonable person skilled in the art, reading the claims in context,
would think he was intending to claim”. He concluded his discussion by quoting
with approval the passages quoted above from Catnic [1983] RPC 183, 243
and Improver [1990] FSR 181, 189, and saying in para 52 that the
principle of purposive construction as Lord Diplock and he had explained it,
gave “effect to the requirements of the Protocol” and was “the bedrock of
patent construction, universally applicable”, whereas the Protocol or Improver
questions were simply “guidelines for applying that principle to
equivalents … , more useful in some cases than in others”.
The approach in the courts of other EPC states
44.
In Germany, the Bundesgerichtshof has stated that a variant will
infringe if (i) “it solves the problem underlying the invention with modified
but objectively equivalent means”, (ii) this would be recognised by the person
skilled in the relevant art, and (iii) that person “focus[sing] on the
essential meaning of the technical teaching protected in the patent” would
regard the variant “as being equivalent to the solution” offered by the
invention - see Case No X ZR 168/00, 2002 GRUR 519 (Schneidmesser I),
para 30. (It is worth noting that in paras 36 to 38 of its judgment in that
case, the Bundesgerichtshof expressly considered the approach which had been
adopted in Catnic [1982] RPC 183 and Improver [1990] FSR 181.)
Judge Meier-Beck of the Bundesgerichtshof, writing extra-judicially (The
Scope of Patent Protection - The Test for Determining Equivalence (2005) 36
IIC 339, 342 to 343) has suggested that the second step involves asking whether
“the person skilled in the art, using his specialist knowledge, [would be] able
to find the modified means at the priority date as having the same effect”,
which he then says has the “meaning that no inventive step is needed”. That
seems to be supported by what was said by the Bundesgerichtshof in Case No X ZR
156/97, 1999 GRUR 977, (Räumschild), paras II.2(c)(aa) and
III.1.
45.
Further guidance as to the German approach to equivalents was very
recently given by the Munich Oberlandesgericht, upholding the decision of the
Landgericht, in Case No 6 U 3039/16 (Eli Lilly & Co v ratiopharm GmbH),
when considering whether pemetrexed ditromethamine infringed the German
equivalent of the Patent in this case. At para II.B.3(a), the Oberlandesgericht
said that in order for “an embodiment that deviates from the literal meaning of
the claim” to be within the scope of protection, “generally three requirements
must be met”. The first was that “the embodiment must solve the problem underlying
the invention with means that are indeed modified, but are objectively
equivalent”. The second requirement was that “the expertise of the person
skilled in the art must enable him to discover the modified embodiment with its
divergent means to be equivalent”. Thirdly, “the thought processes that the
person skilled in the art has to perform in order to do so must be oriented on
the meaning of the teaching protected in the claim”. In para II.B.3(b)(aa), the
Oberlandesgericht suggested that “the decisive factor” was “what individual
effects the features according to the patent … provide in order to attain the
object underlying the claims and whether these effects are achieved through
other means by the [allegedly infringing] embodiment”. The court added that the
doctrine of equivalence would apply to an embodiment “if it not only
essentially achieves the entire effect of the invention, but specifically also
achieves the effect that the feature, which has not been literally implemented,
is supposed to achieve”.
46.
French law, according to the expert witnesses in this case, applies the
doctrine of equivalents where the variant is “different in form but perform[s]
the same function” as the invention, but only where “the function [claimed in
the patent] is a new one”. This seems to be supported by Azéma and Galloux, Droit
de la propriété industrielle, 7th ed (2012), which distinguishes at p 442
between two categories of patents. The first category is those which “in
general terms claim the means that provide for a particular function” (moyens
généraux), or as Arnold J put it in para 160 of his judgment, claims which
cover “general means”. The second category is patents “which indicate the
particular means which infer such function” (moyens particuliers), or
claims which are “narrowly worded to cover specific means” as Arnold J
expressed it. The doctrine is only normally applicable to the first category of
claims. Arnold J added in para 160 that the categorisation of a patent for this
purpose may depend in part on what was known at the priority date - see the
decisions of the Cour de Cassation in Appeal S 09-15668 Institut Pasteur v
Chiron Healthcare, 23 November 2010 and of the Paris Tribunal de Grande
Instance in Case 09/01863 Mundipharma Laboratories GmbH v Sandoz SAS, 2
July 2010.
47.
As Arnold J also explained in para 159 of his judgment, “there is no
need for the claim to be unclear or for it to be widely worded” for the
doctrine of equivalents to be invoked in the French court. Thus, in the
decision of the Cour de Cassation in Appeal No 06-17915 B2M Industries v
Acome, 20 November 2007, “the function of the particular integer that was
said to be infringed pursuant to an equivalent was held to be novel, and
therefore because the means that was said to be equivalent to that integer
performed the same function and produced the result sought by the invention the
means was equivalent to that integer”, to quote from para 161 of Arnold J’s
judgment.
48.
In the Italian courts, the expert witnesses in this case agreed that a
variant would be held to infringe if (i) it reproduced the “inventive core” of
the patent and (ii) it was an obvious variation, although (iii) the fact that
the variant included some modifications which were not obvious and/or the fact
that the variant does not include some of the elements of the patent claim does
not necessarily prevent the variant infringing - see per Arnold J at para 171
of his judgment. This analysis is supported by the Corte di Cassazione
decisions in Case No 257, Forel SpA v Lisec (13 January 2004), Case No
30234, Barilla GER Fratelli SpA v Pastifico Fazion SpA (30 December
2012) and Case No 622, Entsorga Italia Srl v Ecodeco Srl (11
January 2013).
49.
At any rate at local appellate level, Spanish courts appear to have
effectively adopted the approach embodied in the three questions suggested by
Hoffmann J in Improver [1990] FSR 181 - see for instance Laboratorios
Cinfa SA v Eli Lilly & Co Ltd (“Olanzapine”) Court of Appeal of
Barcelona judgment no 8/2008, 17 January 2008.
50.
Following circulation of this judgment in draft, Actavis referred us to
a decision of the Spanish Tribunal Supremo Lundbeck v Cinfa, no
223/2015, 29 April 2015. In the closely reasoned section ELEVEN of its
judgment, the Tribunal Supremo (i) recorded the fact that none of the parties
challenged the approach of the Court below which applied the three Improver questions
(para 5), (ii) stated that the real issue in the case centred on the second
question (para 6), (iii) cast some doubt on the applicability of the Improver
questions in Spanish law (para 10), (iv) disapproved the notion that the
test for obviousness in patentability is necessarily applicable to the second Improver
question (paras 10 and 14), (v) disapproved the notion that, for the second
Improver question to be answered yes, “the skilled person must be
absolutely certain that the variant … would work successfully in resolving the
technical problem faced by the patented invention” (paras 11 and 12), (vi)
preferred instead, a test of “easy to see or comprehend” and “a degree of
predictability” (paras 11 and 18), which involves “a high probability”, rather
than a “reasonable expectation” that the variant would work (paras 15 and 18),
and (vii) concluded on this basis that the Court of Appeal was right to rule that
the allegedly infringing products in that case did not infringe (paras 18 and
19).
51.
As for the Netherlands, helpful guidance may be found in a lecture given
in 2016 by Judge Kalden, the head of the IP division in the Court of Appeal in
The Hague - Article 69 EPC - the Scylla and Charybdis of the European Patent
Convention - Which route did the Dutch courts take? (2016 Symposium German
Bundespatentgericht). She said that, although there have been subtle changes of
emphasis in its decisions, the Supreme Court tends to focus on “the inventive
concept in order to prevent a too literal interpretation of the claims, which
could do injustice to fair protection for the patentee (or lead to an
unnecessary broad interpretation)”. She also explained that the doctrine of
equivalents applies if (i) the variant is “foreseeable at the priority date”,
(ii) “the inventive concept is sufficiently broad to … cover [the] variant”,
(iii) “the variant makes use of - and thus benefits from - the inventive
concept”, and (iv) “reasonable legal certainty [is not thereby] unduly
compromised”. She added that, despite the first condition:
“Variants that are not foreseeable
at the priority date may well, due to later developments, become an obvious
variant at a later date. This may happen in case of a pioneer invention, where
at the priority date the full breadth of the possible applications could or has
not been fully recognised and therefore was not sufficiently taken into account
when drafting a claim. Another possibility is that a new technique becomes
available after the patent was granted, which makes available an obvious
variant. It would be harsh and contrary to fair protection for the patentee to
deny him the right to attack those, again provided such variant falls within
the inventive concept and reasonable legal certainty is taken into account. So
infringement by equivalence is not limited to foreseeable variants only.”
52.
It may be of some significance that the product which Hoffmann J
concluded in Improver [1990] FSR 181 was non-infringing was held by the
German, Italian and Dutch courts to infringe. Of course, the fact that courts
of two states reach different conclusions on the same issue does not of itself
mean that there is a difference in the law of those states, let alone that one
court is wrong and the other right: the evidence may be different, and there
may be issues of judgment on which reasonable judges could differ. However,
consideration of the judgments in those three other courts does suggest a
difference of approach. Thus, in Germany, the Düsseldorf Oberlandesgericht
based its conclusion on the propositions that “a person skilled in the art will
not interpret the coil spring as a spring, but as an elastic body with gaps ...
as it is obvious that the helical spring is not used as a spring per se”, and
that its only essential function, which was shared by the allegedly infringing
product’s slitted rubber rod, was that it could “enter between adjacent areas
of the body (walls), and that the walls must approach it up to clamping it” -
see Epilady Germany II (1993) 24 IIC 838. In Italy, the Milan District Court
held that there was infringement because the slitted rubber rod had structural
characteristics which enabled it to perform the same function in the same way
as the coiled spring referred to in the patent in suit - see Epilady Italy (1992)
Giur Ann Dir Ind, Case No 2823. In the Netherlands, the Gerechtshof upheld the
first instance decision that the allegedly infringing “device embodies an
application of the patented invention, on the grounds that the hair-engaging
component [ie the slitted rubber rod] of the device is a mechanical equivalent
of the helical spring specified in the patent claims”, and the rod was “not
state of the art in the field of depilatory devices” - Epilady Netherlands III
(1993) 24 IIC 832, paras 9 and 11.
The proper approach to infringement claims
53.
Any patent system must strike a balance between the two competing factors
referred to at the end of article 1 of the Protocol, namely “a fair protection
for the patent proprietor [and] a reasonable degree of legal certainty for
third parties”. The balance cannot be struck on an ad hoc case-by-case
basis without any guiding principles, as that would mean that there was no
legal certainty. On the other hand, striking the balance by adopting a normal
approach to interpretation would risk depriving patentees of a proper measure
of protection; as explained in paras 37 to 39 and 52 above, that is clear from
the approach of all the courts which considered the “Epilady” patent, where it
could not seriously have been suggested that, as a matter of language, a
slotted rubber rod falls within the expression “helical metal spring”, even if
one was construing those words in the context of the claim in the patent in
suit. But, if one departs from ordinary language, it is necessary to have some
guidance or to draw some lines, as Lord Hoffmann implied in Kirin-Amgen [2005] RPC 9, para 37. That is why he promulgated his three questions in Improver [1990]
FSR 181, 189. By means of an extended version of the ordinary concept of
“construction” or “interpretation”, Hoffmann J explained how our domestic law,
as laid down in Catnic [1982] RPC 183, implements article 2 of the
Protocol and thus, as I see it, how it gives effect to the doctrine of
equivalents. That approach was (perhaps unsurprisingly) then adopted in Kirin-Amgen
[2005] RPC 9.
54.
In my view, notwithstanding what Lord Diplock said in Catnic [1982]
RPC 183, 242, a problem of infringement is best approached by addressing two
issues, each of which is to be considered through the eyes of the notional
addressee of the patent in suit, ie the person skilled in the relevant art.
Those issues are: (i) does the variant infringe any of the claims as a matter
of normal interpretation; and, if not, (ii) does the variant nonetheless
infringe because it varies from the invention in a way or ways which is or are
immaterial? If the answer to either issue is “yes”, there is an infringement;
otherwise, there is not. Such an approach complies with article 2 of the
Protocol, as issue (ii) squarely raises the principle of equivalents, but
limits its ambit to those variants which contain immaterial variations from the
invention. It is also apparent that the two issues comply with article 1 of the
Protocol in that they involve balancing the competing interests of the patentee
and of clarity, just as much as they seek to balance the encouragement of
inventions and their disclosure with the need for a competitive market. In my
view, issue (i) self-evidently raises a question of interpretation, whereas
issue (ii) raises a question which would normally have to be answered by
reference to the facts and expert evidence.
55.
In Kirin-Amgen [2005] RPC 9, Lord Hoffmann, following his
approach in Improver [1990] FSR 181 (which itself had followed Lord
Diplock’s analysis in Catnic [1982] RPC 183) effectively conflated the
two issues, and indicated that the conflated issue involved a question of
interpretation. I have considerable difficulties with the notion that there is
a single conflated, or compound, issue, and, even if that notion is correct,
that that issue raises a question of interpretation. Indeed, in my view, to
characterise the issue as a single question of interpretation is wrong in
principle, and unsurprisingly, therefore, can lead to error. While normal
principles of interpretation could, I think, accommodate the notion that
“vertically” extended to an item which was not at precisely 90° to another
item, I do not see how such principles could possibly lead to the conclusion
that a slotted rubber rod was within the expression “helical metal spring”. As
Hoffmann J said in Improver [1990] FSR 181, 197, “the angle of the
support member [in the allegedly infringing product in Catnic [1982] RPC
183] can be regarded as an approximation to the vertical”, but “[t]he rubber
rod is not an approximation to a helical spring”. The problem with treating the
issue as one of normal interpretation is thus that that point alone may be
thought to have been sufficient to put an end to the patentee’s infringement
argument on facts such as those in Improver [1990] FSR 181, and there
would seem to have been little purpose in going through the three questions in
that case.
56.
I had wondered whether the question whether issue (ii) truly involves a
question of interpretation raised what was merely an arid issue of
categorisation. However, I have concluded that that nettle needs to be grasped,
because, so long as the issue is treated as one of interpretation, it will lead
to a risk of wrong results in patent infringement cases and it will also lead
to a risk of confusing the law relating to the interpretation of documents. In
my opinion, issue (ii) involves not merely identifying what the words of a
claim would mean in their context to the notional addressee, but also
considering the extent if any to which the scope of protection afforded by the
claim should extend beyond that meaning. As Sir Hugh Laddie wrote in his
instructive article Kirin-Amgen - The End of Equivalents in England? (2009)
40 IIC 3, para 68, “[t]he Protocol is not concerned with the rules of
construction of claims” but with “determining the scope of protection”.
57.
I might add that the notion of a product or process which infringes
despite an immaterial variation from the invention as claimed is by no means
new to domestic patent law. That point is convincingly demonstrated by Sir Hugh
in his article at paras 33 to 39. Thus, in Walton v Potter & Horsfall (1843)
1 WPC 585, Tindal CJ told the jury that they had to decide whether the
defendant’s product was “perfectly distinct” from the patented product, or
whether it varied “only in certain circumstances, which are not material to the
principle and substance of the invention”. And Lord Cairns LC in Clark v
Adie (1877) 2 App Cas 315, 320, referred to the alleged infringer
having “really taken and adopted the substance of the instrument patented”, and
having “taken in substance the pith and marrow of the invention”. The patents
in these cases included relatively primitive forms of claim, but that does not
undermine the fact that our domestic law has long recognised that an immaterial
variation does not get an infringer off the hook. Particularly in the light of
what he said in Catnic [1983] RPC 183, 242, it is worth mentioning that
Lord Diplock himself in Beecham Group Ltd v Bristol Laboratories Ltd [1978]
RPC 153, 200 rejected a submission that “[t]he increasing particularity with
which claims are drafted … has made the doctrine [of pith and marrow]
obsolete”, and said that the doctrine “still remains a part of patent law”.
58.
Turning to the two issues identified in para 54 above, issue (i), as
already mentioned, involves solving a problem of interpretation, which is
familiar to all lawyers concerned with construing documents. While the answer
in a particular case is by no means always easy to work out, the applicable
principles are tolerably clear, and were recently affirmed by Lord Hodge in Wood
v Capita Insurance Services Ltd [2017] 2 WLR 1095, paras 8 to 15. In the
present case, there is no doubt that, according to normal principles of
interpreting documents, the Actavis products do not infringe the Patent, as in
no sensible way can pemetrexed free acid, pemetrexed ditromethamine, or
pemetrexed dipotassium mean, ie be said to fall within the expression,
“pemetrexed disodium” in claim 1 of the Patent, any more than a slotted rubber
rod can be said to be within the expression “a helical metal spring” in the
claim in the Improver patent. According to normal principles of
interpreting documents, then, this would be the end of the matter.
59.
However, the second issue poses more difficulties of principle: what is
it that makes a variation “immaterial”? In that connection, I consider that
Hoffmann J’s three questions in Improver [1990] FSR 181 provide helpful
assistance, a view supported by the fact explained in paras 44 to 52 above that
similar but not identical tests have been adopted in other EPC jurisdictions.
However, each of the three questions requires some exegesis, and, particularly
the second question, some reformulation.
60.
The first Improver question, which asks whether the variant has a
material effect on the way in which the invention works, seems generally
satisfactory. It is a question which was framed in the context of a mechanical
patent, and is not wholly aptly expressed for every type of case. However, in
practice, the question as framed by Hoffmann J, with its emphasis on how “the
invention” works, should correctly involve the court focussing on the “the
problem underlying the invention”, “the inventive core”, or “the inventive
concept” as it has been variously termed in other jurisdictions. In effect, the
question is whether the variant achieves the same result in substantially the
same way as the invention. If the answer to that question is no, then it would
plainly be inappropriate to conclude that it could infringe. If, by contrast,
the answer is yes, then it provides a sound initial basis for concluding that
the variant may infringe, but the answer should not be the end of the matter.
61.
The second Improver question is more problematic. In my view, it
imposes too high a burden on the patentee to ask whether it would have been
obvious to the notional addressee that the variant would have no material
effect on the way in which the invention works, given that it requires the
addressee to figure out for himself whether the variant would work. The facts
of the present case serve to make that proposition good. As Floyd LJ explained
in para 65 of his judgment below, because a chemist “would not be able to
predict the effect of [a] substitution [for the sodium counter-ion] without
testing at least the solubility of the [active ingredient in the Actavis
products]”, it followed that “predicting in advance whether any particular
counter-ion would work was not possible”, and therefore that the second Improver
test could not be answered yes. However, as mentioned in para 25(i) above, salt
screening is a routine exercise in determining suitability, and as Floyd LJ
said, “the chemist would be reasonably confident that he would come up with a
substitute for the sodium counter-ion”. In those circumstances, given that the
inventive concept of the patent is the manufacture of a medicament which
enables the pemetrexed anion to be administered with vitamin B12, it appears to
me that application of the second Improver question fails to accord “a
fair protection for the patent proprietor” as required by article 1 of the
Protocol.
62.
In my opinion, the second question is better expressed as asking
whether, on being told what the variant does, the notional addressee would
consider it obvious that it achieved substantially the same result in
substantially the same way as the invention. In other words, it seems to me
that the second Improver question should be asked on the assumption that
the notional addressee knows that the variant works to the extent that it
actually does work. That, I think, would be a fair basis on which to proceed in
terms of balancing the factors identified in article 1 of the Protocol, and it
is, I think, consistent with the approach of the German, Italian and Dutch
courts. It is also consistent with the fact that the notional addressee is told
(in the patent itself) what the invention does.
63.
This reformulated second question should also apply to variants which
rely on, or are based on, developments which have occurred since the priority
date, even though the notional addressee is treated as considering the second
question as at the priority date. Such an approach is supported by the
desirability of both consistency of approach and pragmatic justice. It seems
right in principle to have the same question, including the same assumption (ie
that the variant works) for all cases. As to pragmatism, the point is touched
on by Judge Kalden in the passage quoted at the end of para 51 above: while the
notional addressee may answer the reformulated second question affirmatively
even where the variant was unforeseeable at the priority date, he is less
likely to do so than in relation to a variant which was unforeseeable as at
that date.
64.
The second test applied by the German courts, as I understand it, at
least sometimes appears to require the variation not to be inventive, but I am
not sure that that is an appropriate requirement, although it is unnecessary to
decide that point on this appeal. If the variation represents an inventive
step, while it may render it less likely that the patentee will succeed on the second
reformulated question, I find it hard to see why that alone should prevent the
resultant variant from infringing the original invention. It may entitle the
infringer to a new patent, in the same way as the invention of a novel use for
a patented invention can itself be patented, but like such a novel use I see no
reason why the variant should not infringe the original patent. Having said
that, it should be added that the German version of the second test will, I
suspect, usually produce the same result as the reformulated second question.
65.
The third Improver question as expressed by Hoffmann J is whether
the notional addressee would have understood from the language of the claim
that the patentee intended that strict compliance with the primary meaning was
an essential requirement of the invention. That is in my view an acceptable
test, provided that it is properly applied. In that connection, I would make
four points. First, although “the language of the claim” is important,
consideration of the third question certainly does not exclude the
specification of the patent and all the knowledge and expertise which the
notional addressee is assumed to have. Secondly, the fact that the language of
the claim does not on any sensible reading cover the variant is certainly not
enough to justify holding that the patentee does not satisfy the third
question. Hence, the fact that the rubber rod in Improver [1990] FSR 181
could not possibly be said to be “an approximation to a helical spring” (to
quote from p 197) was not the end of the infringement issue even in Hoffmann
J’s view: indeed, as I have already pointed out, it was because the rubber rod
could not possibly be said to be a helical spring that the allegedly infringing
product was a variant and the patentee needed to invoke the three Improver questions.
Thirdly, when considering the third question, it is appropriate to ask whether
the component at issue is an “essential” part of the invention, but that is not
the same thing as asking if it is an “essential” part of the overall product or
process of which the inventive concept is part. So, in Improver [1990]
FSR 181, 197, Hoffmann J may have been (and I mean “may have been”) wrong to
reject the notion that “the spring could be regarded as an ‘inessential’”:
while it was undoubtedly essential to the functioning of the “Epilady”, the
correct question was whether the spring would have been regarded by the
addressee as essential to the inventive concept, or inventive core, of the
patent in suit. Fourthly, when one is considering a variant which would have
been obvious at the date of infringement rather than at the priority date, it
is, as explained in para 63 above, necessary to imbue the notional addressee
with rather more information than he might have had at the priority date.
66.
In these circumstances, given the weight that has been given by courts
in this jurisdiction (and indeed in some other jurisdictions) to the three “Improver
questions”, I think it must be right for this court to express in our own
words our reformulated version of those questions. In doing so, it is right to
emphasise, as Lord Hoffmann did in Kirin-Amgen [2005] RPC 9, para 52,
that these questions are guidelines, not strict rules (as indeed the
Oberlandesgericht indicated in Case No 6 U 3039/16, when saying that it was
“generally” true that “three requirements must be met”). While the language of
some or all of the questions may sometimes have to be adapted to apply more
aptly to the specific facts of a particular case, the three reformulated questions
are as follows:
i)
Notwithstanding that it is not within the literal meaning of the
relevant claim(s) of the patent, does the variant achieve substantially the
same result in substantially the same way as the invention, ie the inventive concept
revealed by the patent?
ii)
Would it be obvious to the person skilled in the art, reading the patent
at the priority date, but knowing that the variant achieves substantially the
same result as the invention, that it does so in substantially the same way as
the invention?
iii)
Would such a reader of the patent have concluded that the patentee
nonetheless intended that strict compliance with the literal meaning of the
relevant claim(s) of the patent was an essential requirement of the invention?
In order to establish infringement in a case where there
is no literal infringement, a patentee would have to establish that the answer
to the first two questions was “yes” and that the answer to the third question
was “no”.
Provisional conclusion on direct infringement in the UK
67.
Given that the Actavis products do not infringe on the basis of a normal
interpretation of claim 1 of the Patent, it is necessary to consider whether
they represent an immaterial variation on that claim. I propose to address that
issue initially disregarding the prosecution history, and having reached a
provisional conclusion, I will then address that history and its effect on the
provisional conclusion.
68.
In my view, application in the present case of the three questions just
identified results in the conclusion that the Actavis products infringe. So far
as the first question is concerned, there can be no doubt but that those
products work in the same way as the invention: they all ultimately involve a
medicament containing the pemetrexed anion and vitamin B12. Thus, they achieve
substantially the same result in substantially the same way as the invention.
Indeed, as in the Court of Appeal, Actavis realistically accept that the first question
is to be answered yes.
69.
As to the second question, it seems to me clear that the notional
addressee of the Patent would appreciate (and would have appreciated as at the
priority date) that each of the Actavis products would work in precisely the
same way as pemetrexed disodium when included in a medicament with vitamin B12.
When it comes to different versions of pemetrexed medicaments, it is clear that
the use of a free acid, and of ditromethamine and dipotassium salts was in each
case well established as at the priority date - see para 26(ii) to (iv) above.
Furthermore, the notional addressee of the Patent would regard investigating
whether pemetrexed free acid, pemetrexed ditromethamine or pemetrexed
dipotassium worked as a purely routine exercise - see para 25(i) above. The
reason why I differ from the Court of Appeal and Arnold J on this second
question is that, in accordance with the second question as formulated in Improver
[1990] FSR 181, 189, they considered that the notional addressee should not
be treated as knowing that the Actavis products did in fact work at all, whereas,
as explained above, that seems to me to involve too strict a test.
70.
Turning to the third question, the Court of Appeal considered that the
notional addressee “would understand that the patent was clearly limited to the
disodium salt, and did not extend to the diacid, or the dipotassium or
ditromethamine salts”. They based this conclusion on the fact that the
specification of the Patent contains a number of passages (eg in Para [0022] of
the specification, quoted in para 19 above) which refer to “anti-folates” and
the like and other passages which refer to pemetrexed disodium, which is “a
highly specific chemical compound”, and the fact that the claim is limited to
pemetrexed disodium would therefore lead the notional addressee to conclude
that the claim is indeed intended to be so limited (see paras 71 and 72 of
Floyd LJ’s judgment).
71.
In my opinion, the Court of Appeal adopted an approach which places too
much weight on the words of the claim and not enough weight on article 2 of the
Protocol (and it is only right to add that, in doing so, they were, like Arnold
J at first instance, following Lord Hoffmann’s guidance in Kirin-Amgen [2005] RPC 9). Thus, when considering the third test, Floyd LJ made the point at para
72(ii) of his judgment that “there is no obvious leeway as a matter of language
for giving it a broad as opposed to a narrow construction”. That seems to me to
demonstrate the risk of treating the issue raised by the third question
as being one of normal interpretation. (Another way of looking at the point is,
in the language of Sir Hugh Laddie, that it involves wrongly conflating the
issue of interpretation with the issue of scope of protection.) As already
explained, if it was a decisive point it would make a nonsense of asking the
three questions: if one cannot depart from the language of the claim
when considering those questions, what is the point of the questions in the
first place?
72.
More specifically, I do not agree with the Court of Appeal’s view that,
because the specification referred to “anti-folates” and “anti-folate drugs”,
the fact that the claims were limited to pemetrexed disodium means that the
drafter of the Patent would have been understood to intend that the other
pemetrexed compounds would not infringe. As Mr Mitcheson QC contended in his
well argued case, the point is neutral because there is no reference to
pemetrexed salts as a class in the specification, and the contrast therefore
does not help on the question whether pemetrexed salts other than pemetrexed
disodium were intended to be excluded.
73.
Further, contrary to the Court of Appeal’s reasoning, I would have
thought that if the specification had not referred to anti-folates but had only
referred to pemetrexed disodium, that would have been a more powerful
indication that the patentee was intending to limit himself to pemetrexed
disodium. The very fact that the specification teaches that there are other
anti-folate drugs which have a similar effect to pemetrexed disodium (coupled
with the fact that it was generally known that cations other than sodium could
be successfully used with anti-folates) highlights a point similar to that made
by Lord Diplock in Catnic [1982] RPC 183, 244, namely “No plausible
reason has been advanced why any rational patentee should want to place so narrow
a limitation on his invention” as to limit the scope of protection afforded by
the Patent to pemetrexed disodium - a telling but not always conclusive point. Additionally,
there is no teaching in the specification which relates to the relevance or importance
of the sodium cation.
74.
Looking at matters more broadly, the addressee of the Patent would, as I
see it, understand that the reason why the claims were limited to the disodium salt
was because that was the only pemetrexed salt on which the experiments described
in the specification had been carried out. However, it does not follow that the
patentee did not intend any other pemetrexed salts to infringe: the suggestion
confuses the disclosure of the specification of a patent with the scope of
protection afforded by its claims. Particularly given the facts set out in para
25 above, it seems to me very unlikely that the notional addressee would have
concluded that the patentee could have intended to exclude any pemetrexed salts
other than pemetrexed disodium, or indeed pemetrexed free acid, from the scope
of protection.
75.
Accordingly, I would conclude that, subject to considering the
prosecution history, the Actavis products infringe claim 1 of the Patent.
The effect of the prosecution history
76.
The application for the patent was filed at the EPO in June 2001, and it
contained claims directed to a method of treatment, claims in Swiss form, and
purpose-related product claims. In January 2003, Dr
Burnside, Lilly’s patent attorney, filed a revised set of claims which omitted
the method of treatment claims. Claims 1 and 2 were as follows:
“1. Use of a methylmalonic
acid lowering agent in the preparation of a medicament useful in lowering the
mammalian toxicity associated with an antifolate, and the medicament is administered
in combination with an antifolate.
2. Use of a methylmalonic
acid lowering agent in the preparation of a medicament useful in lowering the
mammalian toxicity associated with an antifolate, and the medicament is
administered in combination with an antifolate and a FBP binding agent.”
Claim 10 was a dependent claim “wherein the antifolate is
ALIMTA”.
77.
As Floyd LJ said, these claims are in the reverse order from the claims
ultimately granted (as they start with the use of the methylmalonic lowering
agent rather than pemetrexed disodium), but nothing hangs on that. The
essential point is that these claims were entirely general as to the identity
of the antifolate. In March 2004, the EPO examiner wrote raising various objections
including some under articles 83 and 84 EPC 2000 (disclosure and clarity). The
clarity and lack of disclosure objections were that the claims related to too
many possible combinations of compounds by using general expressions such as
“antifolate”, “methylmalonic acid lowering agent” and “FBP binding agent”.
Moreover, the examiner was concerned that the claims covered all compounds
having these characteristics or properties, whereas the application provided
support and disclosure for only a very limited number of such compounds.
78.
Dr Burnside replied in a letter of December 2004, under cover of which
he filed new claims 1 and 2, this time starting with the use of the antifolate,
now limited to “pemetrexed” in these terms:
“1. Use of pemetrexed in the
manufacture of a medicament for use in combination therapy for inhibiting tumour
growth in mammals wherein said medicament is to be administered in combination
with vitamin B12 or a pharmaceutical derivative thereof.
2. Use according to claim
1 wherein said medicament is to be administered in combination with vitamin B12
or a pharmaceutical derivative thereof and a folic binding protein binding
agent [which was then defined].”
In support of these new claims, Dr
Burnside said that, “in order to expedite the application proceeding to grant”,
Lilly had elected to amend the claims so as to reflect more closely the
specific examples provided. However, he added, the amendments were made without
prejudice to Lilly’s right to obtain protection for other patentable subject
matter in one or more divisional applications.
79.
Notwithstanding these amendments, in May 2005 the EPO examiner formally
objected to the admissibility of the new claims. He contended that the
amendments introduced subject matter beyond the content of the originally filed
documents, contrary to article 123(2) EPC 2000. Thus, he said, the inclusion in
claim 1 of “use of pemetrexed ...” and similar provisions in other claims did
not find any basis in the application documents as filed. According to the
examiner, “pemetrexed” was a distinct compound from pemetrexed disodium. (This
is supported by the Chemical Abstracts Service Registry, where the “pemetrexed”
is recorded as being the free diacid.) The patent does contain one mention of
the term “pemetrexed” at para [0004] of the specification, followed by a Lilly
reference number which shows it to be pemetrexed disodium. It was therefore, at
best, uncertain as to what the term “pemetrexed” on its own was intended to
refer.
80.
Dr Burnside replied in March 2006 by a letter under cover of which he
filed new claims, which this time were limited to pemetrexed disodium, and are
now embodied in the claims of the Patent as set out in para 21 above. Dr
Burnside said:
“The Claims have been amended to
refer to the preferred embodiment, the use of pemetrexed disodium (ALIMTA®) as
manufactured by Eli Lilly and Company, as the antifolate drug. The Claims have
also been amended to incorporate the list of vitamin B12 derivatives set out on
p 7 lines 6-7 of the application as filed.”
The EPO examiner accepted the claims in
this form, and the application proceeded to grant.
81.
Actavis contends that the prosecution history, as summarised in paras 76
to 80 above, makes it clear that the claims of the Patent should be interpreted
as being limited to pemetrexed disodium not only as a matter of language, but
in the sense that the use of any other pemetrexed compound, including other
pemetrexed salts and the free acid, could not infringe. This contention gives
rise to two issues. The first is one of relatively general application, namely
whether and if so when it is permissible to have recourse to the prosecution
history of a patent when considering whether a variant infringes that patent.
The second issue is whether the prosecution history of the Patent in this case
alters the provisional conclusion reached in para 75 above.
82.
So far as the first issue is concerned, Lord Hoffmann said in Kirin-Amgen
[2005] RPC 9, para 35:
“The courts of the United Kingdom,
the Netherlands and Germany certainly discourage, if they do not actually
prohibit, use of the patent office file in aid of construction. There are good
reasons: the meaning of the patent should not change according to whether or
not the person skilled in the art has access to the file and in any case life
is too short for the limited assistance which it can provide. It is however
frequently impossible to know without access, not merely to the file but to the
private thoughts of the patentee and his advisors as well, what the reason was
for some apparently inexplicable limitation in the extent of the monopoly
claimed.”
83.
In the absence of good reason to the contrary, it would be wrong to
depart from what was said by the House of Lords. It is said by Actavis that
there is good reason to depart from what Lord Hoffmann said on the ground that
he was wrong in his description of the German and Dutch approaches to this
issue, and that anyway he failed to have regard to the jurisprudence of other
European courts.
84.
In my view, Lord Hoffmann was right about the approach of the German and
Dutch courts to this issue. Thus, the Bundesgerichtshof, in a decision
involving the German equivalent of the instant Patent, Case No X ZR 29/15 (Eli
Lilly v Actavis Group PTC), paras 39-40, stated that “it is permissible …
to use statements made by the applicant [and the examiner] during the grant
procedure as an indication of how the person skilled in the art understands the
subject matter of the patent” but “such indications cannot be readily used as
the sole basis for construction”. And in Ciba-Geigy AG v Oté Optics BV (1995)
28 IIC 748, the Dutch Supreme Court said that “a court will only be justified
in using clarifying information from the public part of the granting file, when
it holds that even after the average person skilled in the art has considered
the description and the drawings, it is still open to question how the contents
of the claims must be interpreted”.
85.
It is argued by Actavis that this limited approach to the circumstances
in which reference can be made to the prosecution file may be more restrictive
than the approach adopted in France, Italy, and Spain, as analysed by Arnold J.
Thus, he said in para 162 of his judgment, that the Cour d’Appel observed in
Case No 08/00882, Hewlett Packard GmbH v Agilent Technologies Deutschland
GmbH (27 January 2010) that “the patentee who amended its clauses to give
them a limited scope may not, without putting the safety of third parties at
risk, claim that the amendments were not necessary, nor that the limited claims
have the same scope as the broader claims”. However, the court in that case had
already decided on the natural meaning of the patent, and the contents of the
file were merely being invoked to confirm the decision. The position in Italy,
according to Arnold J in para 174 of his judgment, is that “there is no
doctrine of prosecution history estoppel” and “there is no clear rule as to the
relevance, if any, of the prosecution history as an aid to the interpretation
of claims”. In Spain there is a doctrine of actos propios, which as
Arnold J explained in para 184, is “the doctrine of one’s own acts”, but it
only justifies relying on the prosecution file in relation to statements which
are “unequivocal, clear, precise, conclusive, undoubted and [do] not reflect
any kind of ambiguity”.
86.
While the French courts appear to be more ready to refer to the
prosecution file on issues of interpretation or scope than the German or Dutch
courts, it is unclear how much, if any, difference there is in outcome. The
position in relation to the Italian courts is more unclear, and it may well be
that the effect of the approach of the Spanish courts is the same in outcome as
that of the German and Dutch courts. In those circumstances, particularly as it
may be inevitable that there is a degree of difference in the approach of
different national courts on such an issue, there is nothing in the French,
Italian, or Spanish jurisprudence which causes me to depart from the conclusion
expressed by Lord Hoffmann.
87.
In my judgment, it is appropriate for the UK courts to adopt a sceptical,
but not absolutist, attitude to a suggestion that the contents of the
prosecution file of a patent should be referred to when considering a question
of interpretation or infringement, along substantially the same lines as the
German and Dutch courts. It is tempting to exclude the file on the basis that
anyone concerned about, or affected by, a patent should be entitled to rely on
its contents without searching other records such as the prosecution file, as a
matter of both principle and practicality. However, given that the contents of
the file are publicly available (by virtue of article 128 EPC 2000) and (at
least according to what we were told) are unlikely to be extensive, there will
be occasions when justice may fairly be said to require reference to be made to
the contents of the file. However, not least in the light of the wording of article
69 EPC 2000, which is discussed above, the circumstances in which a court can
rely on the prosecution history to determine the extent of protection or scope
of a patent must be limited.
88.
While it would be arrogant to exclude the existence of any other
circumstances, my current view is that reference to the file would only be
appropriate where (i) the point at issue is truly unclear if one confines
oneself to the specification and claims of the patent, and the contents of the
file unambiguously resolve the point, or (ii) it would be contrary to the
public interest for the contents of the file to be ignored. The first type of
circumstance is, I hope, self-explanatory; the second would be exemplified by a
case where the patentee had made it clear to the EPO that he was not seeking to
contend that his patent, if granted, would extend its scope to the sort of
variant which he now claims infringes.
89.
Turning to the second issue, I do not consider that the contents of the
prosecution file in this case justify departing from the provisional conclusion
expressed in para 75 above. It seems to me clear that the reason why the
examiner considered that the claims in the patent should be limited to
pemetrexed disodium was because the teaching in the specification did not
expressly extend to any other anti-folates. It is unnecessary to decide the
issue, but, at least as at present advised, I am inclined to think that the
examiner was wrong in taking that view. Indeed, in the course of his
well-presented argument for Actavis, Mr Alexander QC seemed to accept that
Lilly could have expressed its claims more widely than it did (albeit that this
was not a point which was carefully explored). However, even if the examiner
was right or at least justified in taking the stance that he did, I do not
consider that that consideration can have any bearing on the question whether
any pemetrexed salts other than pemetrexed disodium should be within the scope
of the patent pursuant to the doctrine of equivalents. The whole point of the
doctrine is that it entitles a patentee to contend that the scope of protection
afforded by the patent extends beyond the ambit of its claims as construed
according to normal principles of interpretation.
90.
This point was well made by the Dutch Court of Appeals in Boston
Scientific Ireland Ltd v Cordis Europa NV 01/639 (unreported) 3 July 2003,
when they held that the contents of the prosecution file were of no assistance,
as they related to a concern which the examiner had expressed about added
matter which went to disclosure, whereas that had no relevance to the point at
issue which was the scope of the claim - which properly included equivalents.
91.
I draw comfort from the fact that neither party was able to refer to a
case where a French or Spanish Court had relied upon the patentee’s response to
a disclosure or added matter objection by the examining officer as being
relevant to the scope of claim. It is true that the Madrid Appeal Court in Inmobiliaria
Masife SL v Vale y Tino SA (decision 268/2013) (unreported) 27 September
2013 held that a patentee was bound by an exclusion which he had agreed during
prosecution but that was “to overcome an objection of the examiner based on the
prior art”, a very different point. I draw even greater comfort from the fact
that the Bundesgerichtshof reached the same conclusion on this very issue in
relation to the German equivalent of the Patent in this case in Case No X ZR
29/15 (Eli Lilly v Actavis Group PTC), para 72.
Direct infringement in France, Italy and Spain
92.
Having concluded that the Actavis products directly infringe the Patent
as a matter of UK law, it is necessary to consider whether the same result
obtains under French, Italian and Spanish law. In my judgment, direct
infringement is established in those jurisdictions as well.
93.
Turning first to French law, it appears to me that the answer to the
question of direct infringement ultimately turns on whether the Patent in this
case falls into the moyens généraux category or the moyens
particuliers category, because, as discussed in para 46 above, the doctrine
of equivalents is apparently only applicable to patent claims in the former
category. With some diffidence, I have reached a different conclusion from
Arnold J on this issue and have concluded that the Patent in this case falls
into the former category. It is of course true that an appellate court should
be very slow indeed to differ from the trial judge on a question of fact.
However, the notion that the resolution of a dispute as to foreign law involves
a factual finding rather than a legal conclusion is somewhat artificial, and in
any event, the Judge did not hear any oral evidence from the expert foreign law
witnesses. We are therefore in as good a position as he was to analyse the
effect of the evidence as to foreign law.
94.
The Judge considered that the Patent in this case represents a moyen
particulier, because pemetrexed disodium was the relevant means and the
Patent did not reveal it having a novel function: it merely revealed a new and
better way in which its function could be achieved. To my mind the better
analysis is that the Patent discloses that pemetrexed disodium could be used
for a function for which it could not previously have been satisfactorily or
safely used in practice; specifically, that pemetrexed disodium could be used
with vitamin B12 to achieve an end which could not have been achieved by either
chemical on its own, pemetrexed disodium because of its harmful side-effects
and vitamin B12 because it would not have worked. The essential point, as I see
it, is that the Patent revealed for the first time the existence of a combined
means which functioned in a certain way, namely to alleviate certain cancers
without serious side-effects. It would be different if the overall function of
the combination of the two chemicals had not been new.
95.
Support for this conclusion appears in the book referred to in para 46
above, Droit de la propriété industrielle, whose two authors were the
expert witnesses on French law in this case. At para 719, p 443, they wrote
“when the claim is over a combination of means for which global function is
novel, any combination of means with a different structure but achieving the
same global function is a priori equivalent and thus infringing”. That passage
was effectively applied by the Cour de Cassation in Appeal P08-14741, Diffusion
Equipements Loisirs v Helge, 15 September 2009.
96.
As to Italian law, Arnold J said at paras 178 and 179 of his judgment
that he had concluded that the Actavis products did not infringe the Italian
designation of the Patent on two grounds. The first (which he only accepted
with “some hesitation”) was “because on its face the patent clearly
demonstrated a conscious intention of the patentee to limit the claims to
pemetrexed disodium”. The second ground was “because if there was any doubt
about that, it was amply confirmed by the prosecution history”. It is clear
that (as one would expect) the Italian courts accept the doctrine of
equivalents, and accordingly for the reasons given in paras 70 to 74 above, I
would reject the first ground; and, for the reasons given in paras 91 to 93
above, I would reject the second ground also.
97.
So far as Spanish law is concerned, it is common ground that the Spanish
courts have followed the United Kingdom approach, which leads to the difficult
question whether one should assume that they would follow this decision in
modifying the Improver questions and in particular the second question.
I incline to the view that judicial comity would tend to suggest that the
Spanish courts would follow this court in modifying the Improver questions,
not least because this appears to render the UK courts and therefore the
Spanish courts more consistent with the German and Dutch courts, and no more
inconsistent with the French and Italian courts.
98.
In a written note dated 10 July 2017, Actavis applied for what would
amount to a reconsideration of the conclusion expressed in para 97 above, on
the ground that the reasoning of the Spanish Tribunal Supremo in the Lundbeck
decision, discussed in para 50 above, should lead to the opposite
conclusion, namely that marketing Actavis’s products in Spain would not
infringe the Patent.
99.
In my view, it is too late for Actavis to raise such an argument. Lilly
had sought to rely on the Lundbeck decision in its written case in this
appeal, and Actavis had objected on the ground that the decision had been given
after the Court of Appeal decision in these proceedings. It seems to me that in
these circumstances it would be wrong to permit Actavis to raise the Lundbeck
decision to support their case, especially as they are seeking to do so
after knowing the result of this appeal and the reasons for that result. I am
unimpressed by Actavis’s argument that their application is nonetheless
justified because the reasoning in para 97 above was not raised on this appeal.
Actavis’s written case stated that “Spanish law has been directly modelled on Catnic
and Improver”, and in paras 182 and 187 of his judgment on this case
Arnold J effectively treated the Improver questions as part of Spanish
law. It appears to me that the conclusion that, if the UK Supreme Court
modifies the Improver questions, the Spanish courts would adopt any such
modification, was therefore within the scope of the argument raised in this
Court.
100.
Furthermore, I consider that it would be wrong for Actavis to be
permitted to raise a new ground in support of their contention that their
products would not infringe in Spain, after publication of our decision, which was
done with their consent and at their instigation following receipt of our draft
judgment which concluded that their products would infringe in Spain. It is not
as if Actavis had come across new information since they had agreed to that
publication. It is true that, as explained in para 2 above, Actavis’s
solicitors wrote to the Court very shortly after they received the draft
judgment, but thereafter they had nearly a full 24 hours within which they
could have withdrawn their agreement to publication of our decision. In any event,
there is obvious force in the simple point that, having agreed to publication
of the decision in advance of the handing down of the judgment, they have to
take the consequences. I do not suggest that, in every case where the decision
is published with the consent of the parties after they have seen the draft
judgment, it would be impossible for either party to invite the court to change
the decision, or any aspect of it. However, it seems to me that, in the absence
of a good reason, the interests of finality and certainty should prevail, and I
do not consider that Actavis have come up with a good enough reason in this
case.
101.
It is right to add that I am by no means convinced that, even if we had
permitted Actavis to re-argue their case in relation to Spain, on the basis of
the Lundbeck decision, I would have reached a different conclusion from
that expressed in para 97 above. Quite what constitutes “a degree of
predictability” or “a high probability” when it comes to assessing whether the
notional addressee would expect the variant to work must be fact-sensitive.
Further, if, as seems likely but not, I accept, certain, the German, Dutch,
French and Italian courts would all hold that Actavis’s products infringed,
there would have been much to be said for the view, which I have already
expressed, that the Spanish courts would follow suit.
102.
Accordingly, I would hold that the French, Italian and Spanish
designations of the Patent are also directly infringed by the Actavis products.
Indirect infringement
103.
In these circumstances, Actavis’s cross-appeal, which seeks to challenge
the Court of Appeal’s conclusion that its products indirectly infringed does
not, I think, arise in the sense that it has no practical effect on the parties
(other, perhaps, than on the issue of costs). However, as the point was fully
argued, gave rise to a disagreement between the Court of Appeal and the trial
judge, and can be dealt with shortly, it is appropriate to consider it.
104.
Indirect infringement is provided for in section 60(2) of the 1977 Act,
and it states that a person infringes a patent if, without the patentee’s
consent, he supplies or offers to supply in the United Kingdom to someone not
authorised by the patentee with “any of the means, relating to an essential
element of the invention, for putting the invention into effect when he knows,
or it is obvious to a reasonable person in the circumstances, that those means
are suitable for putting, and are intended to put, the invention into effect”.
105.
The reason why Lilly contends that, even if they did not directly
infringe, the Actavis products would indirectly infringe is because, when they
are supplied to a doctor or a pharmacist, they are, as Actavis would know,
dissolved in a saline solution in order to enable them to be administered to
patients. Saline is a solution of common salt, ie sodium chloride, in water,
and when common salt is dissolved in water, it separates into sodium cations
and chloride anions. Accordingly, when one of Actavis’s products, say that
containing pemetrexed dipotassium, is dissolved in saline, the solution
contains pemetrexed anions and potassium cations plus sodium cations and
chloride anions. In those circumstances, argues Lilly, even if pemetrexed
dipotassium would not of itself infringe if it was administered with vitamin
B12, at least provided that the ratio of sodium ions to pemetrexed ions was at
least 2:1, there will be infringement when it is administered in saline
solution, because the solution which is administered will contain pemetrexed disodium.
106.
The Court of Appeal, disagreeing with the Judge, acceded to Lilly’s
argument on this point.
107.
Actavis argue that a solution consisting of, or including, pemetrexed
ions and sodium ions is not within the expression “pemetrexed disodium” in the
Patent, because it is limited to the solid, or crystalline, chemical. I agree
with Floyd LJ in rejecting that argument. There is no reason to think that the
patentee intended to limit the expression in that way; quite the contrary. It
is clear that solubility was an important issue, and indeed that was one of the
two main reasons on which Actavis rested their contention that their products
did not infringe, as discussed in paras 24 to 25, 59, and 66 above. Further,
and even more in point, as Floyd LJ said, in the passages quoted in para 19
above the specification made it clear that references to pemetrexed disodium
extended to that chemical in solution.
108.
Actavis also argue that there is an inconsistency between the Court of
Appeal holding, when considering direct infringement, that the notional
addressee could not be assumed to know that pemetrexed dipotassium would
dissolve, and holding, when considering indirect infringement, that pemetrexed
dipotassium did in fact dissolve. Even if I had not concluded that the notional
addressee should be treated as knowing that pemetrexed dipotassium could
dissolve, I would have rejected that argument which seems to me to involve a
non-sequitur. By the time that they were ready to market their products,
Actavis knew perfectly well that they were all soluble.
109.
Actavis further argue that a solution of pemetrexed dipotassium
dissolved in saline does not in any event contain “pemetrexed disodium” within
the meaning of that term in the Patent; it is simply pemetrexed dipotassium
dissolved in saline. In my view that is a bad point. If dissolving pemetrexed
disodium in an aqueous solution of potassium chloride can be said to result in
a solution containing pemetrexed disodium (as Actavis’s argument impliedly
accepts), then it must follow as a matter of elementary chemical logic that
dissolving pemetrexed dipotassium in saline also result in a solution which
contains pemetrexed disodium: the two solutions are chemically identical, as
each would consist of potassium and sodium cations and chloride and pemetrexed
anions in water.
110.
Actavis additionally argue that it is irrational to hold that there
could be indirect infringement because it would all depend on the solvent in
which the Actavis product is dissolved, and, even if that solvent was saline,
it would depend on the proportion of sodium ions and pemetrexed ions in the
solution which would vary by reference to the weight of the patient. The fact
that infringement may depend on the nature of solvent and the relative amounts
of ions in the solution does not seem to me to be irrational. It is simply a
result of the extent of the scope of protection afforded by the patent given
that (as determined by the Court of Appeal) its claims are limited to
pemetrexed disodium, which, when dissolved in water produces two sodium cations
to every one pemetrexed anion.
111.
Finally, Actavis argue that, rather than being used in the manufacture
of a medicament as described in claim 1 of the Patent, pemetrexed disodium is
part of the medicament. Like the Court of Appeal, I do not agree. The pemetrexed
disodium comes into the manufacturing process rather later than it would if the
original medicament included pemetrexed disodium rather than pemetrexed dipotassium,
but that cannot alter the fact that, before it is administered to the patient,
the medicament includes pemetrexed disodium and vitamin B12.
112.
Accordingly, I would uphold the Court of Appeal’s determination that
Actavis are liable to Lilly for indirect infringement in the United Kingdom
with respect to their products if Actavis know, or it is obvious in the
circumstances, that ultimate users will dilute in saline - or at least Actavis
would be liable for indirect infringement if they were not liable for direct
infringement. The Court of Appeal said that this conclusion would apply equally
to France, Italy, and Spain, and there is no challenge to that from Actavis.
Conclusion
113.
For these reasons, I would (i) allow Lilly’s appeal in direct
infringement and hold that the Actavis products infringe the Patent in the
United Kingdom, and also in France, Italy and Spain, (ii) dismiss Actavis’s
cross-appeal on the basis that if its products did not directly infringe, they
would indirectly infringe to the extent held by the Court of Appeal.

