[New search]
[Help]
�
STATUTORY INSTRUMENTS
2005 No. 3281
AGRICULTURE
The Feeding Stuffs (England) Regulations 2005
| |
Made |
28th November 2005 | |
| |
Laid before Parliament |
5th December 2005 | |
| |
Coming into force |
1st January 2006 | |
ARRANGEMENT OF REGULATIONS
PART 1
Introductory and General
PART 2
Presentation and Composition of Feeding Stuffs
PART 3
Enforcement
PART 4
Amendments to other legislation
SCHEDULES
The Secretary of State makes the following Regulations in exercise of the powers conferred by sections 66(1), 68(1) and (1A), 69(1) and (3), 70(1), 71(1), 74(1), 74A, 77(4), 78(6) and (10), 79(1), (2) and (9), and 84 of the Agriculture Act 1970[1] (as read with regulation 14 of the Food Standards Act 1999 (Transitional and Consequential Provisions and Savings) (England and Wales) Regulations 2000[2] and with Articles 2 and 6 of the Ministry of Agriculture, Fisheries and Food (Dissolution) Order 2002)[3].
In so far as these Regulations cannot be made under the powers in the Agriculture Act 1970 specified above, the Secretary of State makes these Regulations in exercise of her powers as a Minister designated[4] for the purposes of section 2(2) of the European Communities Act 1972[5] in relation to the common agricultural policy of the European Community and measures in the veterinary and phytosanitary fields for the protection of public health.
There has been consultation in accordance with the requirements of section 84(1) of the Agriculture Act 1970 or as appropriate of Article 9 of Regulation (EC) No. 178/2002 of the European Parliament and of the Council laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety[6].
PART 1
Introductory and General
Title, commencement and application
1.
These Regulations may be cited as the Feeding Stuffs (England) Regulations 2005, come into force on 1st January 2006 and apply in relation to England only.
Interpretation
2.
—(1) In these Regulations—
"the Act" means the Agriculture Act 1970;
"additive", subject to regulation 21(4), means a feed additive to which the Additives Regulation applies, with the exception of any additive in categories (d) or (e) of Article 6(1) of that Regulation other than those in the functional groups listed in paragraph 4(a), (b) and (c) of Annex 1 to that Regulation;
"the Additives Directive" means Council Directive 70/524/EEC concerning additives in feeding stuffs[7];
"the Additives Regulation" means Regulation (EC) No. 1831/2003 of the European Parliament and of the Council on additives for use in animal nutrition[8];
"ash" means the matter which results from the treatment of a feeding stuff in accordance with the appropriate procedure set out in the method of analysis for ash specified in Point 5 of the Annex to Directive 71/250/EEC[9];
"the Certain Products Directive" means Council Directive 82/471/EEC concerning certain products used in animal nutrition[10];
"complementary feeding stuff" means a compound feeding stuff which has a high content of certain substances and which, by reason of its composition, is sufficient for a daily ration only if it is used in combination with other feeding stuffs;
"complete feeding stuff" means a compound feeding stuff which, by reason of its composition, is sufficient for a daily ration;
"compound feeding stuff", subject to regulation 14(6), means a mixture of feed materials, whether or not containing any additive, for oral feeding to pet animals or farmed creatures in the form of complementary feeding stuffs or complete feeding stuffs;
"the Compound Feedingstuffs Directive" means Council Directive 79/373/EEC on the circulation of compound feedingstuffs[11];
"daily ration" means the average total quantity of feeding stuff, expressed on a 12% moisture basis, required daily by an animal of a given kind, age group and level of production in order to satisfy all its nutritional needs;
"EEA State" means a Member State, Norway, Iceland or Liechtenstein;
"energy value" means the energy value of a compound feeding stuff calculated in accordance with the relevant method specified in Schedule 1;
"establishment" has the meaning given by Article 3(d) of Regulation (EC) No. 183/2005 of the European Parliament and of the Council laying down requirements for feed hygiene[12];
"fat" means the extract obtained following the treatment of a feeding stuff in accordance with the appropriate procedure set out in the method of analysis for oils and fats specified in Part IV of the Annex to Directive 71/393/EEC[13];
"feeding stuff intended for a particular nutritional purpose" means a compound feeding stuff, the composition or method of manufacture of which distinguishes it from other feeding stuffs and from the type of products covered by Council Directive 90/167/EEC laying down the conditions governing the preparation, placing on the market and use of medicated feeding stuffs in the Community[14], and in respect of which any indication is given that it is intended for a particular nutritional purpose;
(a) any product of vegetable or animal origin, in its natural state, fresh or preserved;
(b) any product derived from such a product by industrial processing; or
(c) any organic or inorganic substance,
(whether or not containing any additive) and for use in oral feeding to pet animals or farmed creatures, directly as such, or after processing, in the preparation of a compound feeding stuff or as a carrier of a premixture;
"the Feed Materials Directive" means Council Directive 96/25/EC on the circulation of feed materials[15];
"fibre" means the organic matter calculated following the treatment of a feeding stuff in accordance with the procedure set out in the method of analysis for fibre specified in Point 3 of Annex 1 to Directive 73/46/EEC[16];
"mammalian meat and bone meal" has the meaning given in Regulation 3(1) of the TSE (England) Regulations 2002[17];
"Member State" means a Member State other than the United Kingdom;
"micro-organism" has the meaning given by Article 2(2)(m) of the Additives Regulation;
"milk replacer feed" means a compound feeding stuff administered in dry form, or after reconstitution with a given quantity of liquid, for feeding young animals as a supplement to, or substitute for, post-colostral milk or for feeding calves intended for slaughter;
"mineral feeding stuff" means a complementary feeding stuff which is composed mainly of minerals and which contains at least 40% by weight of ash;
"minimum storage life" means, in relation to a compound feeding stuff, the date until which, under proper storage conditions, that feeding stuff retains its specific properties;
"molassed feeding stuff" means a complementary feeding stuff prepared from molasses and which contains at least 14% by weight of total sugar expressed as sucrose;
"moisture" means water and other volatile material determined in accordance with the procedure set out in the method of analysis for moisture specified in Part I of the Annex to Directive 71/393/EEC[18];
"oil" means the extract obtained following the treatment of a feeding stuff in accordance with the appropriate procedure set out in the method of analysis for oils and fats specified in Part IV of the Annex to Directive 71/393/EEC[19];
"particular nutritional purpose" means the purpose of satisfying any nutritional requirement of pet animals or productive livestock, the process of assimilation or absorption of which, or the metabolism of which, may be temporarily impaired, or is temporarily or permanently impaired, and which may therefore benefit from ingestion of a feeding stuff capable of achieving that purpose;
"pet food" means a feeding stuff for pet animals and "compound pet food" shall be construed accordingly;
"premixture" has the meaning given by Article 2(2)(e) of the Additives Regulation, excluding any premixture consisting solely of feed additives in categories (d) or (e) of Article 6(1) of that Regulation, other than those in the functional groups listed in paragraph 4(a), (b) and (c) of Annex 1 to that Regulation;
"prescribed material" means material described in regulation 5(1);
"product intended for animal feed" means any product used or intended for use in feed for pet animals, farmed creatures or animals living freely in the wild;
"protein", except in paragraphs 7(2), 8, 9 and 10 of Part I of Schedule 3 where it has the meaning given to it by regulation 3(1) of the TSE (England) Regulations 2002[20], means the matter obtained as a result of treatment of a feeding stuff in accordance with the procedure set out in the method of analysis for protein specified in Point 2 of Annex 1 to Directive 72/199/EEC[21];
"put into circulation" means sell or otherwise transfer, have in possession with a view to selling or otherwise transferring, or offer for sale, in each case to a third party, and in regulations 13(8) and 14 also means import into England from a state other than an EEA State;
"starch" means the matter obtained as the result of treatment of a feeding stuff in accordance with the procedure set out in the method of analysis for starch specified in Point 1 of Annex 1 to Directive 72/199/EEC[22];
"2000 Regulations" means the Feeding Stuffs Regulations 2000[23];
"undesirable substance" means any substance or product, not being a pathogenic agent, which is present in or on a product intended for animal feed and—
(a) constitutes a potential danger to animal or human health or the environment; or
(b) could adversely affect livestock production.
(2) Any reference in these Regulations to a numbered regulation or Schedule shall, unless the context otherwise requires, be construed as a reference to the regulation or Schedule bearing that number in these Regulations.
(3) Where, in any tabular or other entry in a Schedule to these Regulations, a numbered reference to a footnote appears, the footnote so numbered shall be treated as included in or amplifying the text to which it relates.
(4) Any reference in these Regulations to a numbered section shall, unless otherwise indicated, be construed as a reference to the section bearing that number in the Act.
(5) Any reference in these Regulations to a Community instrument shall be construed as a reference to that instrument as amended on the date that these Regulations are made.
Modification of the Agriculture Act 1970 in relation to all feeding stuffs
3.
—(1) Subsection (1) of section 66 shall have effect in England as if—
(a) for the definition of "feeding stuff" there were substituted the following definition—
(a) a product of vegetable or animal origin in its natural state (whether fresh or preserved);
(b) a product derived from the industrial processing of such a product; or
(c) an organic or inorganic substance, used singly or in a mixture,
whether or not containing additives, for oral feeding to pet animals or farmed creatures;";
(b) for the definition of "pet animal" there were substituted the following definition—
"
"pet animal" means an animal belonging to a species normally nourished and kept, but not consumed, by man, other than an animal bred for fur;".
(2) Subsection (2) of section 66 shall have effect in England as if the following were substituted for paragraph (b) of that subsection—
(3) Sections 73 and 73A shall have effect in England as if, for the words "animals of any description prescribed for the purpose of the definition of "feeding stuff" in section 66(1) of this Act" there were substituted the words "any farmed creatures".
(4) Section 85 shall have effect in England as if—
(a) in so far as it relates to delivery outside the United Kingdom, paragraph (a) were omitted; and
(b) paragraph (b) were omitted.
Modification of the Agriculture Act 1970 in relation to imported feeding stuffs
4.
In relation to feeding stuffs which have been imported, section 69(1) shall have effect in England as if the words "and in either case before it is removed from the premises" were omitted.
Prescribed material to which requirements for the statutory statement and marking apply
5.
—(1) Subject to paragraph (2), the material prescribed for the purposes of sections 68(1) and 69(1) is any material usable as a feeding stuff.
(2) For the purposes of these Regulations section 68(2) does not apply.
Exemption from these Regulations
6.
In so far as provisions of these Regulations implement the Compound Feedingstuffs Directive (which principally regulates the labelling and packaging of compound feeding stuffs), they shall not apply in the circumstances specified in Article 14(c) (relating to animals kept for scientific or experimental purposes) of that Directive.
Revocations
7.
The Feeding Stuffs Regulations 2000, with the exception of regulation 19A and paragraph 19 of Schedule 4 of those Regulations, are revoked in relation to England, together with the amending instruments listed in Schedule 9 and to the extent specified in that Schedule.
PART 2
Presentation and Composition of Feeding Stuffs
Matters required and permitted to be contained in a statutory statement or otherwise declared
8.
Except in respect of additives and premixtures not contained in feeding stuffs, the particulars, information and instructions required or permitted to be contained in a statutory statement or otherwise declared, are as specified in and shall comply with the provisions of Schedule 3.
Forms of statutory statement
9.
—(1) Except in the circumstances relating to small quantities of feeding stuffs mentioned in Article 5(2) of the Compound Feedingstuffs Directive and subject to paragraph (2), the statutory statement—
(a) in the case of any prescribed material delivered in a package or other container, shall—
(i) take the form of a label attached to that package or container; or
(ii) be clearly marked directly on the package or container, and
(b) in the case of such material delivered in bulk, shall take the form of a document relating to and accompanying each consignment.
(2) In the case of any feed material sold in a quantity not exceeding 10 kg, and supplied directly to the final user, the statutory statement may be provided in the form of a notice in writing.
(3) The particulars, information and instructions required or permitted to be contained in the statutory statement shall—
(a) be clearly separate from any other information;
(b) subject to paragraphs (5) and (6), be in English; and
(c) be legible and indelible.
(4) For the purposes of section 69 (marking of material prepared for sale), prescribed material which is contained in a package or other container shall be labelled or marked in the manner prescribed in relation to such material in paragraph (1)(a) or, where applicable, (2), and such material in bulk shall be marked by the display in as close proximity to the material as may be practicable of a document relating thereto.
(5) In the case of any compound feeding stuff or feed material which is intended for export to a Member State, the statutory statement shall be in one or more official Community languages, as determined by that Member State.
(6) In the case of any feeding stuff, except a feeding stuff containing an additive in category (d) or (e) of Article 6(1) of the Additives Regulation other than those in the functional groups listed in paragraph 4(a), (b) or (c) of Annex 1 to that Regulation, which is intended for export to an EEA State that is not a Member State, the statutory statement shall be in one or more of the official languages of the country of destination.
Limits of variation
10.
—(1) Section 74(2) shall have effect in England as if after the words "this Part of this Act" there were inserted the words "or the Feeding Stuffs (England) Regulations 2005".
(2) For the purposes of section 74, as modified by paragraph (1), the limits of variation in relation to any mis-statement in a statutory statement, document or mark, as to the nature, substance or quality of a feeding stuff where the mis-statement relates to—
(a) any analytical constituent specified in the first column of—
(i) Part A of Schedule 4 (where the feeding stuff is a compound feeding stuff not intended for pet animals),
(ii) Part B of Schedule 4 (where the feeding stuff is a compound pet food), or
(iii) Part C of Schedule 4 (in the case of a feed material);
(b) any vitamin or trace element specified in the first column of Part D of that Schedule; or
(c) the energy value of any feeding stuff specified in the first column of Part E of that Schedule,
shall be as set out with respect to that constituent or vitamin, trace element or feeding stuff, in the corresponding entry in the second Column of the relevant Part of that Schedule.
(3) Particulars with respect to any material which are contained in a statutory statement, or in any document, or which are marked on, or denoted by a mark on, the material, shall not, for the purposes of Part IV of the Act or of these Regulations, be treated as false by reason of any mis-statement therein as to the nature, substance or quality of the material, if—
(a) the material was first sold, or otherwise put into circulation in an EEA State;
(b) the mis-statement did not, at the time of putting into circulation, exceed any limits of variation prescribed in relation thereto in the State concerned; and
(c) any such limits were in accordance with any applicable European Community legislation.
Assigned meanings for statutory statements or marks
11.
For the purposes of section 70, there is assigned to the expressions "complementary feeding stuff", "complete feeding stuff", "compound feeding stuff", "milk replacer feed", "mineral feeding stuff" and "molassed feeding stuff" in each case the meaning given by regulation 2(1) to the expression concerned.
Manner of packaging and sealing compound feeding stuffs
12.
—(1) Subject to paragraphs (2) and (3), no person shall put into circulation a compound feeding stuff, unless it is in a bag or container, and that bag or container is sealed in such a way that, when the bag or container is opened, the seal is damaged and cannot be re-used.
(2) Compound feeding stuffs may be put into circulation in bulk, in unsealed bags or in unsealed containers, in the case of—
(a) deliveries between producers of compound feeding stuffs or those putting them into circulation;
(b) deliveries from producers of compound feeding stuffs to packaging enterprises;
(c) compound feeding stuffs obtained by mixing grain or whole fruit;
(d) blocks or licks;
(e) small quantities not exceeding 50 kg in weight, which are intended for the final user and are taken directly from a bag or container which, before opening, complied with the sealing provision in paragraph (1).
(3) Compound feeding stuffs may be put into circulation in bulk, or in unsealed containers, but not in unsealed bags, in the case of—
(a) direct deliveries from the producer to the final user;
(b) molassed feeding stuffs consisting of less than three feed materials;
(c) pelleted feeding stuffs.
Control of feed materials
13.
—(1) In this regulation any reference to a numbered Part means a Part of Schedule 2.
(2) No person shall put into circulation any feed material of a description specified in column (3) of Part II under a name other than that specified in the corresponding entry in column (2) of that Part.
(3) No person shall put into circulation any feed material not listed in Part II under a name specified in column (2) of that Part or under a name or term which could otherwise mislead a purchaser as to the real identity of the material.
(4) Where the name of a feed material listed in column (2) of Part II includes a common name or term listed in column (4) of Part I no person shall put into circulation any such feed material or any compound feeding stuff containing such feed material unless the feed material was prepared by the process specified in columns (2) and (3) of Part I corresponding to that common name or term.
(5) No person shall put into circulation any feed material or any compound feeding stuff containing any feed material, unless—
(a) in the case of any feed material of a description specified in column (3) of Part II the botanical purity by weight of the feed material is not less than the percentage (if any) specified in relation to it in column (3) of Part II or, if none is specified, is not less than 95% ; or
(b) in the case of any feed material of a description specified in column (1) of Part III the botanical purity by weight of the feed material is not less than 95% ; and
the feed material also complies with the provisions regarding botanical and chemical purity set out in paragraph 1 of Section II of Part A of the Annex to the Feed Materials Directive.
(6) No person shall use any feed material to bind another feed material, if the quantity of the feed material so used exceeds 3% of the total weight of the feed material bound.
(7) Without prejudice to sections 73 and 73A, no person shall import into England from any state which is not an EEA State, supply (otherwise than on sale), have in possession with a view to so supplying or use any feed material which is deleterious or dangerous to farmed creatures, to pet animals or, through consumption of the products of any animal fed with the feed material, to human beings.
(8) No person shall put into circulation or use any feed material which presents a danger to the environment.
(9) No person shall put into circulation any feed material in a manner likely to mislead as to its properties.
(10) In paragraph (5)(a) "description" shall be taken to exclude any botanical purity requirement, and for the purposes of this regulation and of Schedule 2 "botanical purity" shall be construed in accordance with paragraph 2 of Section II of Part A of the Annex to the Feed Materials Directive.
Control of products intended for animal feed containing undesirable substances
14.
—(1) No person shall—
(a) put into circulation any product intended for animal feed which is specified in column 2 of Schedule 5; or
(b) use any such product for animal feed,
if it contains any undesirable substance specified in column 1 of that Schedule in excess of the level specified for it in column 3 of that Schedule.
(2) No person shall put into circulation, or use as a feeding stuff, any complementary feeding stuff if—
(a) having regard to the quantity of it recommended for use in a daily ration, it contains any undesirable substance specified in column 1 of Schedule 5 in excess of the level specified for it in column 3 of that Schedule in relation to complete feeding stuffs; and
(b) there is no provision relating to any complementary feeding stuff in the corresponding entry in column 2 of that Schedule.
(3) No person shall mix any product intended for animal feed which is specified in column 2 of Schedule 5 and which contains any undesirable substance specified in column 1 of that Schedule in excess of the level specified for it in column 3 of that Schedule for the purpose of dilution with any product intended for animal feed.
(4) No person shall put into circulation any product intended for animal feed or use any such product for animal feed unless it is—
(a) sound and genuine; and
(b) of merchantable quality.
(5) For the purposes of paragraph (4), a product intended for animal feed which is specified in column 2 of Schedule 5 is not sound, genuine and of merchantable quality if it contains any undesirable substance specified in column 1 of that Schedule in excess of the level specified in relation to it in column 3 of that Schedule.
(6) For the purposes of paragraph (2) "feeding stuff" includes feeding stuffs for oral feeding to animals living freely in the wild, and "complementary feeding stuff" and "complete feeding stuff" shall be construed accordingly.
(7) Paragraph (8) shall apply to any person who has in his possession or control for the purpose of a trade or business any of the following products intended for animal feed—
(a) palm kernel expeller;
(b) feeding stuffs obtained from the processing of fish or other marine animals;
(c) seaweed meal and feed materials derived from seaweed; or
(d) complete feeding stuffs for fish or for fur producing animals.
(8) Any person referred to in paragraph (7) shall, if requested by an inspector, procure and produce to the inspector an analysis in order to demonstrate that the content of inorganic arsenic in a product intended for animal feed listed in paragraph (7) is within the limit specified in the relevant entry in column 3 of Schedule 5.
(9) Any person who without reasonable excuse fails to comply with a request made under paragraph (8) shall be guilty of an offence and liable on summary conviction to a fine not exceeding level 3 on the standard scale.
Control of feeding stuffs containing prohibited materials
15.
—(1) No person shall put into circulation for use as a feeding stuff, or use as a feeding stuff, any material which contains—
(a) faeces, urine or separated digestive tract contents resulting from the emptying or removal of the digestive tract, irrespective of any form of treatment or admixture;
(b) hide treated with tanning substances, including its waste;
(c) seeds or other plant propagating materials which, after harvest, have undergone specific treatment with plant protection products for their intended propagation, or derived by-products;
(d) wood, sawdust or other materials derived from wood which has been treated with wood preservatives as defined in Annex V to Directive 98/8/EC of the European Parliament and of the Council concerning the placing of biocidal products on the market[25];
(e) subject to paragraph (3), waste (whether or not subjected, or to be subjected, to further processing) obtained from the treatment of "urban waste water", "domestic waste water" or "industrial waste water" (as those terms are defined in Article 2 of Council Directive 91/271/EEC concerning urban waste water treatment), whatever the origin of the waste water concerned[26];
(f) solid urban waste, such as household waste, but excluding catering waste as defined by Regulation (EC) 1774/2002 of the European Parliament and of the Council laying down health rules concerning animal by-products not intended for human consumption[27];
(g) packaging and parts of packaging from products used in agriculture or the food industry.
(2) For the purposes of paragraph (1) "waste" has the meaning given in Article 1 of Council Directive 75/442/EEC on waste[28].
(3) For the purposes of paragraph 1(e), the term "waste water" shall be construed in accordance with the footnote to point 5 of the Annex to Commission Decision 2004/217/EC establishing a list of materials whose circulation or use for animal nutrition purposes is prohibited[29].
Control of certain protein sources
16.
—(1) Subject to paragraphs (3) and (4), no person shall sell or have in possession with a view to sale, for use as a feeding stuff or as a protein source in a feeding stuff, any material belonging to a product group specified in column 1 of Schedule 6, unless that material—
(a) is named as a permitted product in column 2 of that Schedule; and
(b) complies with all the specifications and requirements contained in and imposed in relation thereto by columns 3 to 6 of that Schedule.
(2) Subject to paragraph (3), no person shall sell or have in possession with a view to sale for use as a feeding stuff, or use as a feeding stuff, any product obtained from yeasts of the "Candida" variety cultivated on n-alkanes.
(3) Paragraphs (1) and (2) do not apply in relation to any material or product excluded from application of the Certain Products Directive by Article 16 thereof concerning exports to third countries.
(4) Paragraph (1) does not apply in the circumstances authorised for derogation by Article 3(2) (concerning scientific or experimental purposes) of the Certain Products Directive.
Control of the iron content of milk replacer feeds
17.
No person shall put into circulation any milk replacer feed intended for calves of up to 70 kilograms live weight, which has an iron content of less than 30 milligrams per kilogram of the complete feeding stuff at a moisture content of 12% .
Control of ash insoluble in hydrochloric acid in compound feeding stuffs
18.
—(1) No person shall put into circulation—
(a) any compound feeding stuff composed mainly of rice by-products in which the level of ash insoluble in hydrochloric acid exceeds 3.3% of its dry matter; or
(b) subject to paragraph (2), any other compound feeding stuff in which the level of ash insoluble in hydrochloric acid exceeds 2.2% of its dry matter.
(2) Paragraph (1)(b) shall not apply to the putting into circulation of any compound feeding stuff which—
(a) contains permitted mineral binders named or described in the Annex to Commission Directive 2003/57/EC[30];
(b) is a mineral feeding stuff;
(c) contains more than 50% of sugar beet chips or sugar beet pulp; or
(d) is intended for farmed fish and has a fish meal content of more than 15% ,
if the level of ash insoluble in hydrochloric acid is declared in the statutory statement as a percentage of the feeding stuff as such.
Control of feeding stuffs intended for particular nutritional purposes, and supplementary provisions relating to statutory statement
19.
—(1) No person shall put into circulation any feeding stuff intended for a particular nutritional purpose unless—
(a) the particular nutritional purpose in question is specified in column 1 of Chapter A of Schedule 7;
(b) the feeding stuff possesses the essential nutritional characteristics specified opposite that particular nutritional purpose in column 2 of that Chapter;
(c) the feeding stuff is intended for animals specified opposite that particular nutritional purpose in column 3 of that Chapter;
(d) it is recommended that the feeding stuff be used for a period of time falling within the range specified opposite that particular nutritional purpose in column 5 of that Chapter;
(e) in relation to the feeding stuff, the requirements specified in paragraphs 1, 2 and 8 of Chapter B of Schedule 7 are complied with; and
(f) the composition of the feeding stuff is such that it is capable of achieving the particular nutritional purpose for which it is intended.
(2) Schedule 7 shall have effect as specified in Schedule 3.
Control of additives and premixtures
20.
—(1) No person shall contravene or fail to comply with the provisions of the Additives Regulation specified in paragraph (2).
(2) The provisions referred to in paragraph (1) are—
(a) Article 3 (placing on the market, processing and use of feed additives), paragraphs (1) to (4), as read with Article 10 (status of existing products);
(b) Article 12 (supervision);
(c) Article 16, paragraphs (1) to (5), (labelling and packaging of additives and premixtures).
(3) In any proceedings for an offence under paragraph (2)(a) it shall be a defence to prove that the act giving rise to the offence—
(a) is one to which Article 10 of the Additives Regulation applies; and
(b) would not have constituted an offence under the 2000 Regulations as they were immediately before the coming into force of these Regulations.
(4) In any proceedings for an offence under paragraph (2)(c) it shall be a defence to prove that the act giving rise to the offence—
(a) is one to which Article 25(2) of the Additives Regulation applies; and
(b) would not have constituted an offence under the 2000 Regulations as they were immediately before the coming into force of these Regulations.
(5) Notwithstanding the revocation referred to in regulation 7, where before the 18th October 2004 initial comments had been forwarded to the Commission in accordance with regulation 11(2) of the 2000 Regulations that application shall be treated as if regulation 11 of those Regulations were still in force.
Saving relating to confidential information relating to additives under the 2000 Regulations
21.
—(1) Notwithstanding the revocations in regulation 7, and subject to paragraphs (2) and (3), no person shall publish or disclose any confidential information that was, prior to the coming into force of these Regulations, obtained by him in the performance of functions under regulation 11 of the 2000 Regulations without the previous consent in writing of—
(a) the person who, in accordance with that regulation, made an application for a Community authorisation of, or as the case may be, for a new use of, the additive concerned; or
(b) that person's assignee or successor to ownership of the confidential information.
(2) Nothing in paragraph (1) shall restrict the publication or disclosure of such information for the purpose of the exercise of functions under that regulation.
(3) Nothing in paragraph (1) shall prevent the publication or disclosure of confidential information of a type specified in Article 7(2) of the Additives Directive.
(4) In this regulation, "confidential information" means information of the type specified in Article 7(1) of the Additives Directive and "additive" has the meaning given in Article 2 of that Directive.
(5) A publication or disclosure in contravention of paragraph (1) shall be punishable as if it were a disclosure prohibited by section 83.
PART 3
Enforcement
Enforcement of provisions made under section 2(2) of the European Communities Act 1972
22.
In so far as any provision of these Regulations is made under section 2(2) of the European Communities Act 1972, that provision shall be enforced as if it were made under those provisions of Part IV of the Act under which the other provisions of these Regulations are made, and the provisions of that Part shall apply accordingly.
Modification of section 74A(3) of the Agriculture Act 1970
23.
—(1) For the purposes of the enforcement and administration of the provisions specified in paragraph (2), section 74A(3) shall have effect as if for the words "imposed by regulations under subsection (1) above, or fails to comply with any other provision of the regulations," there were substituted the words "or fails to comply with any requirement imposed by any provision specified in regulation 23(2) of the Feeding Stuffs (England) Regulations 2005".
(2) The provisions specified for the purposes of paragraph (1) are regulation 12(1), 13(2) to (9), 14(1) to (4), 15(1), 16(1) and (2), 17, 18(1), 19(1) and 20(1).
PART 4
Amendments to other legislation
Amendments to the Feeding Stuffs (Sampling and Analysis) Regulations 1999
24.
—(1) The Feeding Stuffs (Sampling and Analysis) Regulations 1999[31] are amended in relation to England in accordance with paragraphs (2) to (4).
(2) In Schedule 2 Part I (general provisions), in sub-paragraph 3(e)(ii) for the words "listed in Schedule 7" to the end of the sub-paragraph substitute the expression "listed in Schedule 5 to the Feeding Stuffs (England) Regulations 2005".
(3) In Annex 1 to Part II of Schedule 2 for the second entry for Starch (polarimetric method) substitute—
(a) in column 2 the words "Point 1 of Annex 1 to Directive 72/1999/EEC (as replaced entirely by the Annex to Directive 1999/79/EC)";
(b) in column 3 the words "OJ No. L123, 29.5.72, p. 6 (OJ/SE 1966-1972 supplement p. 74) OJ No. L209, 7.8.99, p. 23".
(4) In Schedule 3 Part II (notes for completion of certificate), in sub-paragraph (a) of note (11)—
(a) omit the expression "as amended" through to and including the expression "Feeding Stuffs (Enforcement) (Amendment) (England) (No. 2) Regulations 2003";
(b) for the expression "Feeding Stuffs Regulations 2000" substitute "Feeding Stuffs (England) Regulations 2005".
Signed by authority of the Secretary of State for Health
Caroline Flint
Parliamentary Under Secretary of State, Department of Health
28th November 2005
SCHEDULE 1Regulation 2(1) and Schedule 3
METHOD OF CALCULATING THE ENERGY VALUE OF COMPOUND FEEDS
The energy value of compound poultry, ruminant and pig feeds and feeding stuffs intended for particular nutritional purposes for cats and dogs shall be calculated in accordance with the relevant formulae set out below, on the basis of the percentages of certain analytical components of the feed. After application of these formulae, the results shall be given to one decimal place.
Poultry feeds: megajoules (MJ) of metabolisable energy (ME), nitrogen corrected, per kilogram of compound feed.
MJ of ME/kg of feed = 0.1551 × % protein(¹) + 0.3431 × % oil(²) + 0.1669 × % starch(³) + 0.1301 × % total sugar (expressed as sucrose)(4).
Ruminant feeds: megajoules (MJ) of metabolisable energy (ME) per kilogram of dry matter in the compound feed.
MJ of ME/kg of dry matter = 0.14 × % Neutral detergent Cellulase plus Gamanase Digestibility(5) + 0.25 × % oil(²).
Pig feeds: megajoules (MJ) of digestible energy (DE) per kilogram of dry matter in the compound feed.
MJ of DE/kg of dry matter = 17.47 + 0.079 × % protein(¹) + 0.158 × % oil(²) − 0.331 × % ash(6) − 0.140 Neutral Detergent plus Amylase Fibre(5).
(NB) Where the results of analysis are to be given on a dry matter basis, this may be achieved by analysing either the dried material, or fresh material and correcting for the moisture content.
(¹) Determined by the method of analysis for protein specified in Point 2 of Annex 1 to Directive 72/199/EC[32].
(NB) For pig feed the results must be corrected to 100% dry matter.
(²) Determined by the appropriate procedure set out in the method of analysis for oils and fats specified in Part IV of the Annex to Directive 71/393/EEC[33].
(NB) In ruminant and pig feeds the result must be corrected to 100% dry matter.
(³) Determined by the method of analysis for starch specified in Point 1 of Annex 1 to Directive 72/199/EEC[34].
(4) Determined by the method of analysis for sugar specified in Point 12 of the Annex to Directive 71/250/EEC[35].
(5) Determined by the method detailed in the booklet "Prediction of Energy Values of Compound Feeding Stuffs for Farm Animals" (originally published by the Ministry of Agriculture, Fisheries and Food Publications, now available from the Department of the Environment, Food and Rural Affairs under reference No. PB1285).
(6) Determined by the method of analysis for ash specified in Point 5 of the Annex to Directive 71/250/EEC[36].
(NB) The result must be corrected to 100% dry matter.
SCHEDULE 2Regulation 2(1) and 13 Schedule 3 Part I paragraphs 7 and 20
CONTROL OF FEED MATERIALS
PART
I
PRINCIPAL PROCESSES USED FOR THE PREPARATION OF THE FEED MATERIALS LISTED IN PART II OF THIS SCHEDULE


PART
II
NON-EXCLUSIVE LIST OF THE MAIN FEED MATERIALS


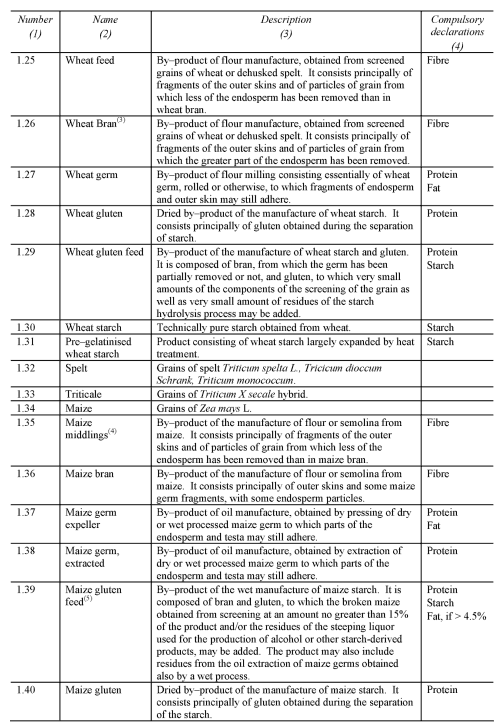



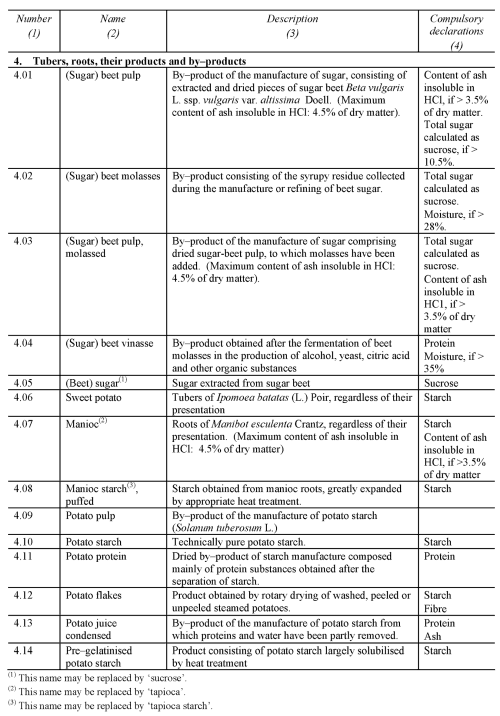





PART
III
OTHER FEED MATERIAL

SCHEDULE 3Regulation 8
CONTENTS OF THE STATUTORY STATEMENT OR OTHER DECLARATION (EXCEPT FOR ADDITIVES AND PREMIXTURES NOT CONTAINED IN FEEDING STUFFS)
PART
I
Interpretation
1.
The expression "in the case of any compound feeding stuff", wherever it appears in this Schedule, shall be construed as referring to any compound feeding stuff which is put into circulation.
Additive declarations (applicable to all feeding stuffs)
2.
Where any person puts into circulation any feeding stuff to which there has been added in the course of manufacture or preparation for putting into circulation, an additive of any of the kinds specified below (other than as an authorised medicated pre-mix or an authorised intermediate product within the meaning of Council Directive 90/167/EEC[37]) and which is not excluded from application of the Additives Directive by Article 22 of that Directive (concerning exports to third countries), the following particulars shall be contained in the statutory statement—
(a) for antioxidants, colourants or preservatives—
(i) if the feeding stuff is a compound feeding stuff other than a pet food, the name of the additive;
(ii) if the feeding stuff is a pet food and it is not covered by paragraph (iii) below, the words "with antioxidant", "coloured with" or "colourant", or "preservative" or "preserved with", as appropriate, followed by the name of the additive; and
(iii) if the feeding stuff is a pet food, it is put up in a package having a net weight not exceeding 10 kilograms, its statutory statement contains a reference number by means of which the feeding stuff concerned may be identified, and its manufacturer supplies, on request, details of the name of the additive concerned,—
(aa) the particulars specified in paragraph (ii) above, or
(bb) the words "with antioxidant", "coloured with" or "preserved with", as appropriate, followed by the word "EC additives";
(b) for vitamin A, D or E, the name of the vitamin, and the active substance level (in the case of vitamin A or D) or the alpha-tocopherol level as acetate (in the case of vitamin E), whether naturally present or added, together in either case with an indication of the period during which that level will remain present but where more than one of these vitamins is present, either the period for each or only the shortest of such periods;
(c) for copper, the name of the additive and the total level of the element, whether naturally present or added;
(d) for enzymes—
(i) the names of the active constituents according to their enzymatic activities, as specified in the authorisation concerned;
(ii) the identification number allotted by the International Union of Biochemistry;
(iii) the activity units (expressed as activity units per kilogram or activity units per litre);
(iv) an indication of the period during which the activity units will remain present;
(v) an indication of any significant characteristics of the enzyme arising during manufacture, as specified in the authorisation concerned; and
(vi) the EC registration number;
(e) for micro-organisms—
(i) the identification of each strain, in accordance with the authorisation;
(ii) the file number of each strain;
(iii) the number of colony-forming units (expressed as CFU/kg);
(iv) the EC registration number;
(v) an indication of the period during which the colony-forming units will remain present; and
(vi) an indication of any significant characteristics of the micro-organisms arising during manufacture, as specified in the authorisation concerned.
3.
In relation to the additives specified below the following particulars may be contained in the statutory statement in addition to those required by paragraph 2—
(a) for trace elements other than copper (if the amount present can be determined by the method of analysis specified in Point 3 of the Annex to Directive 78/633/EEC[38] or by some other valid scientific method), the name of the additive and the total level of the element, whether naturally present or added; and
(b) for vitamins other than vitamins A, D and E, provitamins and substances having a similar chemical effect (if the amount present can be determined by any valid scientific method), the name of the additive, the active substance level, whether naturally present or added, and an indication of the period during which that level will remain present.
4.
Any amount referred to—
(a) in paragraph 2(c), (3)(a) or 3(b) shall be expressed in milligrams per kilogram; and
(b) in paragraph 2(b) shall be expressed in million international units per kilogram, international units per kilogram, milligrams per kilogram or micrograms per kilogram, as appropriate.
5.
By way of exception to paragraph 4(a), any amount referred to in paragraph 2(c), 3(a) or 3(b) may be expressed as a percentage by weight, unless the amount is less than 0.1% by weight, in which case it shall be expressed in milligrams per kilogram or micrograms per kilogram as appropriate.
6.
The particulars required or permitted by paragraphs 2 or 3 to be included in the statutory statement may be accompanied (in the case of any additive not being an enzyme or a micro-organism) by the trade name or the EC registration number of any additive named therein.
Warning statements
7.
Where any person puts into circulation any feed material comprising protein derived from mammalian tissue but containing no mammalian meat and bone meal, and intended for animals other than pet animals, the statutory statement shall contain the following declaration—
"
This feed material comprises protein derived from mammalian tissue the feeding of which to ruminants is prohibited".
8.
Where any person puts into circulation any feed material comprising or containing mammalian meat and bone meal, and intended for animals other than pet animals, the statutory statement shall contain the following declaration—
"
This feed material comprises protein derived from mammalian tissue the feeding of which to ruminants, all other categories of farmed creatures and equine animals is prohibited".
9.
In the case of any compound feeding stuff containing protein derived from mammalian tissue but containing no mammalian meat and bone meal, and intended for animals other than pet animals, the statutory statement shall contain the following declaration—
"
This compound feeding stuff contains protein derived from mammalian tissue the feeding of which to ruminants is prohibited".
10.
In the case of any compound feeding stuff containing mammalian meat and bone meal, and intended for animals other than pet animals, the statutory statement shall contain the following declaration—
"
This compound feeding stuff contains protein derived from mammalian tissue the feeding of which to ruminants, all other categories of farmed creatures and equine animals is prohibited."
Feed materials
11.
Subject to paragraphs 12 to 15, in the case of any feed material which is put into circulation by any person the following particulars shall be contained in the statutory statement—
(a) in the case of any feed material of a kind specified in column (3) of Part II to Schedule 2—
(i) the corresponding name specified in column (2) of that Part (the inclusion of any word appearing in brackets in that column being optional); and
(ii) the particulars (if any) specified in relation to the feed material in the corresponding entry in column (4) of that Part;
(b) in the case of any feed material of a kind specified in column (1) of Part III to Schedule 2—
(i) its name or description there specified, or a name and description (other than one specified in that column, or in column (2) of Part II to that Schedule) sufficiently specific to indicate the nature of the material, and in conformity with the criteria specified in the Introductory Notes to Part II to that Schedule; and
(ii) the particulars specified in relation to the feed material in the corresponding entry in column (2) of Part III to that Schedule;
(c) in the case of any feed material—
(i) subject to regulation 9(5) as read with Article 6(4) of the Feed Materials Directive, which shall be observed where applicable, the words "feed material";
(ii) the moisture content of the feed material, if it exceeds 14% by weight of the feed material or, where a different percentage is specified in relation to that feed material in Part II or Part III to Schedule 2, if it exceeds that percentage;
(iii) the moisture content of the feed material, where it does not exceed the relevant percentage specified in paragraph (ii), but a purchaser requests that the moisture content be declared;
(iv) the level of ash soluble in hydrochloric acid in the feed material, if that level exceeds 2.2% in the dry matter or, where a different percentage is specified in relation to that feed material in Part II or Part III to Schedule 2, if it exceeds that percentage;
(v) where any other feed material has been used to denature the feed material, the nature and quantity of the other feed material so used;
(vi) where any other feed material has been used to bind the feed material, the nature of the other feed material so used;
(vii) the net quantity of the feed material, expressed in units of mass in the case of any solid feed material and, in the case of any liquid feed material, in units of mass or volume;
(viii) where the feed material is part of a divided batch of feed materials, reference to the original batch;
(ix) the name or business name, and the address or registered business address, of the person within the European Community responsible for the particulars specified in this sub-paragraph, if the establishment referred to in sub-paragraph (x) is not responsible for them; and
(x) where the establishment producing the feed material must be approved in accordance with Regulation (EC) No 1774/2002 of the European Parliament and of the Council laying down health rules concerning animal by-products not intended for human consumption[39]; the name or business name, and the address or registered business address, of the establishment, the approval number, the batch reference number or any other particulars which ensure that the material can be traced.
12.
The particulars specified in paragraph 11(a)(ii) and (b)(ii) and (c)(ii) to (iv) shall not be required where—
(a) before the feed material concerned is supplied, the person to whom it is supplied notifies the supplier in writing that those particulars need not be supplied, or
(b) any feed material of animal or vegetable origin, fresh or preserved, and intended for pet animals, is supplied (in a quantity not exceeding 10 kg) directly to the final user thereof, by a person established in the United Kingdom.
13.
—(1) In the case of any feed material which—
(a) originated in a third country, and
(b) is, for the first time, put into circulation in England and the European Community,
in the circumstances specified in the introductory paragraph of Article 6(2) of the Feed Materials Directive, provisional details of the particulars specified in paragraph 11(a)(ii), (b)(ii) and (c)(ii) to (iv) may be provided, if the requirements of sub-paragraph (2) below are observed.
(2) The requirements of this sub-paragraph are observed if—
(a) the person responsible for giving those particulars gives notification, in advance, of the impending arrival of the feed material in England, to an inspector appointed under section 67(3) by the authority which, by virtue of section 67(1), has the duty to enforce Part IV of the Act at the intended place of arrival;
(b) the provisional details are accompanied by the following declaration in bold type—
"
provisional data to be confirmed by . . . . . . . . . . . . . . . . . . . . (name and address of the laboratory instructed to carry out the analyses) regarding . . . . . . . . . . . . . . . . . . . . (reference number of the sample to be analysed) before . . . . . . . . . . . . . . . . . . . . date;" and
(c) the person responsible as mentioned in subparagraph (a) provides the final particulars in question to the person to whom the feed material is supplied, and to the inspector referred to in sub-paragraph (a), within 10 days of its arrival in England.
(3) Where the requirements of sub-paragraph (2) are observed, it shall be the duty of the inspector concerned to notify the European Commission that, in relation to the feed material concerned, the provisional particulars concerned have been provided, and to inform the Commission of the nature of those particulars.
14.
—(1) The particulars specified in paragraph 11 shall not be required in the case of any feed material of animal or vegetable origin, in its natural state, fresh or preserved, and which is not treated with an additive other than any preservative, if the feed material is provided by a farmer-producer to a breeder-user, both of whom carry on business in the United Kingdom.
(2) For the purposes of this paragraph, "farmer-producer" and "breeder-user" shall have the same meaning as in the Feed Materials Directive.
15.
—(1) The particulars specified in paragraph 11(a)(ii), (b)(ii), and (c)(ii) to (vii) shall not be required in the case of any feed material which is a by-product of vegetable or animal origin derived from agro-industrial processing, and which has a moisture content greater than 50% .
(2) For the purposes of this paragraph, "agro-industrial processing" shall have the same meaning as in the Feed Materials Directive.
16.
—(1) Subject to sub-paragraph (2), in the case of any feed material which is put into circulation by any person, information may be provided in addition to the particulars required or permitted to be contained in the statutory statement or otherwise declared.
(2) Any such information provided in addition to the particulars required or permitted to be contained in the statutory statement or otherwise declared—
(a) shall be clearly separated from those particulars;
(b) shall relate to objective or quantifiable factors which can be substantiated; and
(c) shall not be misleading.
Compound feeding stuffs: general
17.
—(1) Subject to sub-paragraph (2), in the case of any compound feeding stuff, the following particulars shall be contained in the statutory statement—
(a) the description "complete feeding stuff", "complementary feeding stuff", "mineral feeding stuff", "molassed feeding stuff", "complete milk replacer feed" or "complementary milk replacer feed" as appropriate;
(b) the species or category of animal for which the feeding stuff is intended and directions for the proper use of the feeding stuff, indicating the purpose for which it is intended, except where the feeding stuff is constituted from no more than three ingredients and is clearly described by reference to its ingredients, either in the statutory statement or elsewhere on its package, label or container; and
(c) the name or trade name and address or registered office of the person established in the European Community responsible for the accuracy of the particulars which, in accordance with this Schedule are required in the case of compound feeding stuffs to be contained in the statutory statement or otherwise declared.
(2) In the case of—
(a) any pet food, the descriptions "complete pet food" and "complementary pet food" may be used instead of "complete feeding stuff" and "complementary feeding stuff" respectively;
(b) any feeding stuff for pet animals other than dogs or cats, each of the descriptions "complete feeding stuff" and "complementary feeding stuff" may be replaced by either of the descriptions "compound feeding stuff" or "compound pet food", but in such a case the statutory statement shall comply with paragraph 19 below and the provisions relating to complete feeding stuffs in Part II of this Schedule, even if it would not otherwise be required to do so.
18.
In the case of any compound feeding stuff, the following particulars shall be declared either in the statutory statement, or elsewhere on the package, label or container (in which case the statutory statement shall indicate where they are to be found)—
(a) the net quantity, expressed in the case of solid products in units of mass, and in the case of liquid products in units of mass or volume;
(b) the minimum storage life, which shall be expressed—
(i) in the case of microbiologically highly perishable feeding stuffs, by the words "use before . . ." followed by the appropriate date (day, month and year), and
(ii) in all other cases by the words "best before . . ." followed by the appropriate date (month and year),
except that, where an expiry date for a period is required to be declared by paragraph 2(b) or 3(b), and is earlier than the appropriate date otherwise required by this paragraph, that expiry date shall be used as the appropriate date;
(c) the batch reference number; and
(d) the approval or registration number allocated by the relevant enforcement authority to the establishment which manufactured the compound feeding stuff.
19.
—(1) In the case of any compound feeding stuff other than a whole grain mix, the statutory statement—
(a) shall include such declarations of the matters provided for in the columns of Part II of this Schedule as must be included; and
(b) may include such declarations provided for in the columns of Part II of this Schedule as may be included,
for consistency with Article 5 of the Compound Feedingstuffs Directive.
(2) In the case of a whole grain mix which is put into circulation, the statutory statement may include such of the declarations provided for in the columns of Part II of this Schedule as may be included for consistency with Article 5 of the Compound Feedingstuffs Directive.
20.
—(1) In the case of any compound feeding stuff other than a whole grain mix, the moisture content shall be declared in the statutory statement if it exceeds the following levels—
|
milk replacer feeds and other compound feeding stuffs with a milk product content exceeding 40% |
7% |
|
mineral feeding stuffs containing no organic substances |
5% |
|
mineral feeding stuffs containing organic substances |
10% |
|
other compound feeding stuffs |
14% |
(2) In the case of a whole grain mix, or a compound feeding stuff with a moisture content not exceeding the limits stated in sub-paragraph (1) which is put into circulation, the moisture content may be declared in the statutory statement.
21.
In the case of any compound feeding stuff having a level of ash insoluble in hydrochloric acid not exceeding the relevant level specified in regulation 18(1)(a) or, as the case may be, (b), that level may be declared in the statutory statement as a percentage of the feeding stuff as such.
22.
In the case of any compound feeding stuff, the following particulars may be included in the statutory statement—
(a) if the manufacturer is not the person responsible for the labelling particulars, the name or business name and the address or registered business address of the manufacturer;
(b) an indication of the physical condition of the feeding stuff or the specific processing it has undergone;
(c) the date of manufacture, expressed as follows—
"
manufactured … [days, months or years] before the minimum storage life expiry date indicated … [place where indicated if not on statutory statement].";
(d) the identification mark or trade mark of the person responsible for the particulars which, in accordance with this Schedule, are required or permitted in the case of compound feeding stuffs to be contained in the statutory statement or otherwise declared;
(e) the description or trade name of the feeding stuff;
(f) the price of the feeding stuff; and
(g) the country of origin or manufacture of the feeding stuff.
23.
—(1) In the particulars required or permitted by paragraphs 18 to 21 and 25 and by paragraph 19 of Schedule 4 to the 2000 Regulations to be set out in the statutory statement—
(a) unless the paragraph in question specifies some other method of expression, the amounts shown shall be expressed in each case as a percentage of the weight of the feeding stuff as such; and
(b) phosphorus shall be expressed as "phosphorus P".
(2) An expression of an amount as being within a range of percentages set out in the statutory statement shall not be regarded as compliance with sub-paragraph (1).
24.
—(1) Subject to sub-paragraph (2), in the case of any compound feeding stuff, information may be provided in addition to the particulars required or permitted to be contained in the statutory statement or otherwise declared.
(2) Any information provided pursuant to sub-paragraph (1)—
(a) shall be clearly separated from those particulars;
(b) shall not be designed to indicate the presence or content of analytical constituents other than those the declaration of which is provided for in this Schedule or in Schedule 7;
(c) shall relate to objective or quantifiable factors which can be substantiated;
(d) shall not be misleading, in particular by attributing to the feeding stuff effects or properties that it does not possess, or by suggesting that it possesses special characteristics, when all similar feeding stuffs contain similar properties;
(e) shall not claim that the feeding stuff will prevent, treat or cure a disease;
(f) shall not, in the case of any feeding stuff intended for a particular nutritional purpose, include a generic description other than in the form of the generic term "dietetic";
(g) shall not, in the case of any feeding stuff other than one intended for a particular nutritional purpose, include a generic description in that form; and
(h) shall not include reference to a particular pathological condition, unless—
(i) the feeding stuff is intended for a particular nutritional purpose, and
(ii) the particular nutritional purpose is specified in respect of that feeding stuff in column 1 of Chapter A of Schedule 7 and relates to that condition.
Compound pet food: specific provisions
25.
—(1) In the case of any compound feeding stuff for dogs or cats, all the feed materials shall be declared in the statutory statement.
(2) In the case of any compound feeding stuff for pet animals other than dogs and cats, the feed materials may be declared in the statutory statement, and in such case all the feed materials shall be declared.
(3) Subject to paragraph 29(2) below and paragraph 3 of Chapter B of Schedule 7, feed materials declared in accordance with sub-paragraph (1) or (2) above shall be declared either—
(a) by their specific names, with an indication of the amount of each feed material; or
(b) by their specific names in descending order by weight; or
(c) by categories, as described in Part I of Schedule 8, in descending order by weight,
and the use of one of those forms of declaration shall preclude the use of either of the others, except—
(i) where the declaration is by categories and any feed material belongs to none of the categories described in Part I of Schedule 8, in which case that feed material, designated by its specific name, shall be listed in order by weight in relation to the categories; or
(ii) in the case of any feeding stuff intended for a particular nutritional purpose, paragraph 29(2) below and paragraph 3 of Chapter B of Schedule 7 require the declaration of any feed material by its specific name, in which case any feed material to which those provisions do not apply may be declared by reference to the category to which it belongs.
26.
Where any declaration under paragraph 25 or under paragraph 19 of Schedule 4 to the 2000 Regulations is by specific names, any feed material described in column 3 of Part II of Schedule 2 shall be declared by the corresponding name specified in column 2 of that Part (the inclusion of any word appearing in brackets in that column being optional).
Complementary feeding stuffs
27.
—(1) In the case of any complementary feeding stuff which, subject to Article 10 of the Additives Regulation, is put into circulation and contains any additive in excess of the maximum content in relation to complete feeding stuffs specified for that additive in the relevant Part of Parts I to VIII of the Table to Schedule 3 to the 2000 Regulations or, as the case may be, in the relevant European Community Regulation, and which is not covered by Article 22 (concerning exports to third countries) of the Additives Directive, the instructions for use in the statutory statement shall state, according to the species and age of the animal, the maximum quantity in grams or kilograms of the feeding stuff which, under these Regulations, may be given per animal per day, and shall be so formulated that, when they are correctly followed, the final content of the additive in relation to complete feeding stuffs does not exceed the maximum so specified in relation to them.
(2) Sub-paragraph (1) shall not apply to any products delivered to manufacturers of compound feeding stuffs or to their suppliers.
Ingredients to which particular attention is drawn
28.
—(1) Subject to sub-paragraph (2), in the case of any compound pet food, or of any feeding stuff intended for a particular nutritional purpose for animals other than pet animals which is put into circulation, particular attention may be drawn in the statutory statement, or elsewhere on the package, label or container, to the presence or low content of one or more ingredients which are essential aspects of the characteristics of the feeding stuff.
(2) Where particular attention is drawn to the presence or low content of any ingredient, as permitted by sub-paragraph (1), the minimum or maximum content, expressed in terms of the percentage by weight of that ingredient, shall be clearly indicated—
(a) opposite the statement which draws attention to that presence or low content;
(b) in the list of ingredients; or
(c) by mentioning that presence or low content and the percentage thereof (by weight) opposite the corresponding category of ingredients.
Feeding stuffs for particular nutritional purposes
29.
—(1) Subject to sub-paragraph (2), in the case of any feeding stuff intended for a particular nutritional purpose which is put into circulation, the following particulars shall be contained in the statutory statement—
(a) the term "dietetic";
(b) a description of the feeding stuff;
(c) the particular nutritional purpose of the feeding stuff, as specified in column 1 of Chapter A of Schedule 7;
(d) the essential nutritional characteristics of the feeding stuff, as specified in column 2 of that Chapter;
(e) the declarations prescribed in column 4 of that Chapter;
(f) the declarations, if any, prescribed in column 6 of that Chapter;
(g) where any declarations prescribed in that column do not include a declaration that it is recommended that the prior opinion of a veterinarian be sought, the words "It is recommended that a specialist's opinion be sought before use"; and
(h) the recommended length of time for use of the feeding stuff.
(2) The particulars required by sub-paragraph (1) to be contained in the statutory statement shall be declared in accordance with the requirements of paragraphs 3–7 and 9 of Chapter B of Schedule 7.
30.
—(1) Subject to sub-paragraph (2), in the case of any feeding stuff intended for a particular nutritional purpose which is put into circulation, particular attention may be drawn in the statutory statement, or elsewhere on the package, label or container, to the presence or low content of one or more analytical constituents which are essential aspects of the characteristics of the feeding stuff.
(2) Where particular attention is drawn to the presence or low content of any analytical constituent, as permitted by sub-paragraph (1), the maximum or minimum content, expressed in terms of the percentage by weight of that analytical constituent, shall be clearly indicated in the list of analytical constituents.
Permitted protein products
31.
—(1) In the case of any product named as a permitted product in column 2 of Schedule 6, the statutory statement shall contain, in addition to any other particulars required by these Regulations, the name specified for that product in column 7 of that Schedule, together with such further particulars as may be specified in that column in relation to it.
(2) In the case of any compound feeding stuff containing, for use as a protein source, any product named as a permitted product in column 2 of Schedule 6, the statutory statement shall contain, in addition to any other particulars required by these Regulations, the name specified for that product in column 7 of that Schedule, together with such further particulars as may be specified in that column in relation to compound feeding stuffs containing that product.
PART
II
DECLARATION OF ANALYTICAL CONSTITUENTS
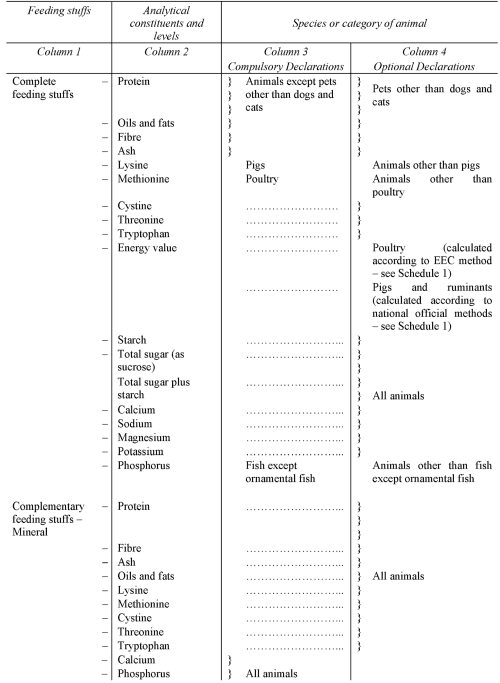

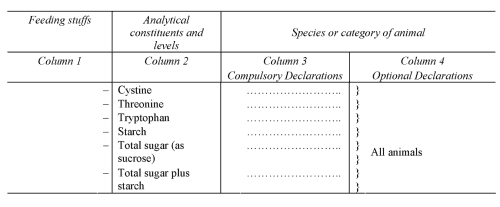
SCHEDULE 4Regulation 10
LIMITS OF VARIATION
PART
A
COMPOUND FEEDING STUFFS EXCEPT THOSE FOR PETS




PART
B
COMPOUND PET FOODS




PART
C
FEED MATERIALS



PART
D
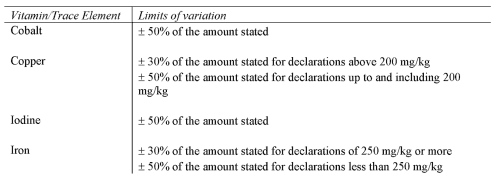
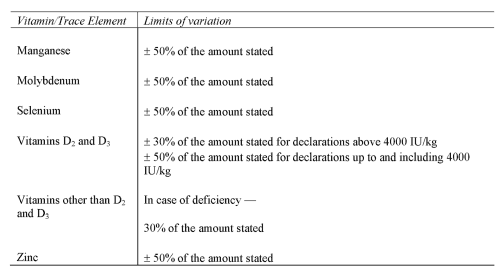
VITAMINS AND TRACE ELEMENTS
PART
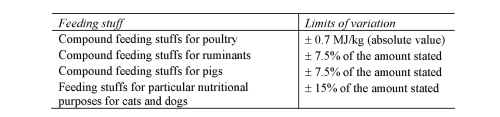
E
ENERGY VALUE OF COMPOUND FEEDING STUFFS
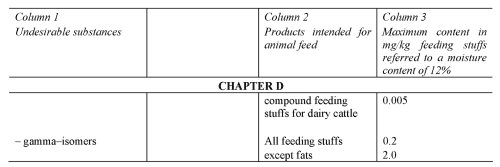
SCHEDULE 5Regulation 14
PRESCRIBED LIMITS FOR UNDESIRABLE SUBSTANCES








SCHEDULE 6Regulation 16 and Schedule 3 Part I, paragraph 29
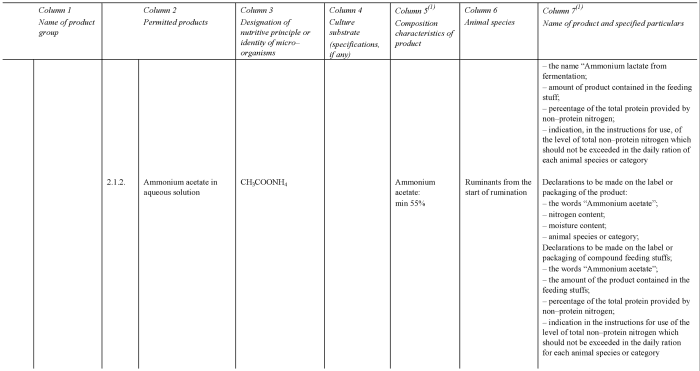
CONTROL OF CERTAIN PROTEIN SOURCES





SCHEDULE 7Regulation 19 and Schedule 3 Part I, paragraphs 18, 25 and 30
PERMITTED FEEDING STUFFS INTENDED FOR PARTICULAR NUTRITIONAL PURPOSES AND PROVISIONS RELATING TO THEIR USE









 CHAPTER B
CHAPTER B
1.
Where there is more than one group of nutritional characteristics indicated in column 2 of Chapter A, denoted by "and/or", for the same nutritional purpose, the feeding stuff may have either or both groups in order to fulfil the nutritional purpose specified in column 1.
2.
Where a group of additives is mentioned in column 2 or column 4 of Chapter A, the additive(s) used must be authorised as corresponding to the specified essential characteristic.
3.
Where the source(s) of feed materials or of analytical constituents is/are required in column 4 of Chapter A the manufacturer must make a specific declaration (i.e. specific name of the feed material(s), animal species or part of the animal) allowing the evaluation of conformity of the feeding stuff with the corresponding essential nutritional characteristics.
4.
Where the declaration of a substance, also authorised as an additive, is required by column 4 of Chapter A and is accompanied by the expression "total", the declared content must refer to, as appropriate, the quantity naturally present where none is added or the total quantity of the substance naturally present and the amount added as an additive.
5.
The declarations specified in column 4 of Chapter A which include the words "if added" are required where the feed material or the additive has been incorporated or its content increased specifically to enable the achievement of the particular nutritional purpose.
6.
The declarations to be given in accordance with column 4 of Chapter A concerning analytical constituents and additives must be expressed in quantitative terms.
7.
The recommended period of use indicated in column 5 of Chapter A indicates a range within which the nutritional purpose should normally be achieved. Manufacturers may refer to more precise periods of use, within the permitted range.
8.
Where a feeding stuff is intended to meet more than one particular nutritional purpose, it must comply with the corresponding entries in Chapter A.
9.
In the case of a complementary feeding stuff intended for a particular nutritional purpose, guidance on the balance of the daily ration must be provided in the instructions for use.
SCHEDULE 8Regulation 8 and Schedule 3 Part I, paragraph 18
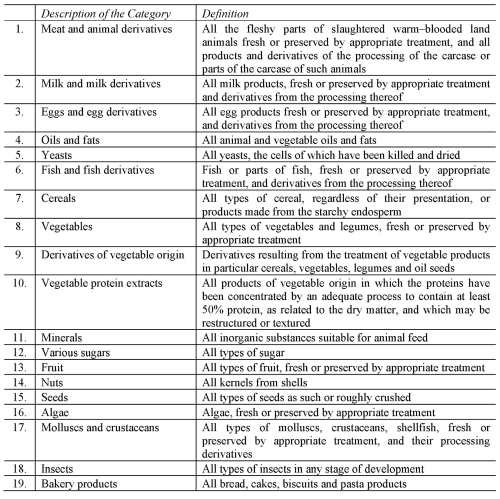
CATEGORIES OF FEED MATERIALS FOR USE IN RELATION TO COMPOUND FEEDING STUFFS FOR PET ANIMALS
SCHEDULE 9Regulation 7
AMENDING INSTRUMENTS REVOKED
The Feeding Stuffs and the Feeding Stuffs (Enforcement) (Amendment) (England) Regulations 2001[40], in so far as they amend the 2000 Regulations.
The Feeding Stuffs (Amendment) Regulations 2002[41], in so far as they amend the 2000 Regulations in relation to England.
The Feeding Stuffs (Amendment) Regulations 2003[42], in so far as they amend the 2000 Regulations in relation to England.
The Feeding Stuffs, the Feeding Stuffs (Sampling and Analysis) and the Feeding Stuffs (Enforcement) (Amendment) (England) Regulations 2003[43], with the exception of regulations 6 and 10(c), in so far as they amend the 2000 Regulations.
The Feeding Stuffs, the Feeding Stuffs (Sampling and Analysis) and the Feeding Stuffs (Enforcement) (Amendment) (England) (No.2) Regulations 2003[44], in so far as they amend the 2000 Regulations.
The Feeding Stuffs, the Feeding Stuffs (Sampling and Analysis) and the Feeding Stuffs (Enforcement) (Amendment) (England) Regulations 2004[45] in so far as they amend the 2000 Regulations.
The Feeding Stuffs, the Feeding Stuffs (Sampling and Analysis) and the Feeding Stuffs (Enforcement) (Amendment) (England) (No.2) Regulations 2004[46] in so far as they amend the 2000 Regulations.
EXPLANATORY NOTE
(This note is not part of the Regulations)
1.
These Regulations which apply in relation to England only—
(a) largely revoke and replace the Feeding Stuffs Regulations 2000 as amended, ("the 2000 Regulations");
(b) introduce new provisions to enforce and administer Regulation (EC) No. 1831/2003 on additives for use in animal nutrition ("the Additives Regulation"); and
(c) implement Commission Directive 2004/116/EC amending the Annex to Council Directive 82/471/EEC as regards the inclusion of Candida guilliermondii.
They provide for the implementation or as the case may be the continuing implementation of the EC Directives and Decision listed at paragraph 11 below.
2.
The Regulations apply to farmed creatures and pet animals, and in regulation 14 also to animals living freely in the wild.
3.
The Regulations preserve the modifications made to the Agriculture Act 1970 ("the Act") by regulations 20 and 21 of the 2000 Regulations, with the minor drafting amendment that the definition of "pet animal" is made explicit rather than by reference to EC legislation (regulations 3 and 4).
4.
They continue to prescribe the material that is "prescribed material" for the purposes of sections 68(1) and 69(1) of the Act as any material useable as a feeding stuff (regulation 5). Under those sections, sellers of prescribed materials are required to give to purchasers "statutory statements" covering the composition of the material and information on storage, handling and use. Material held for sale must be marked with such information.
5.
They revoke with certain exceptions the provisions of the 2000 Regulations, which were last amended by S.I. 2004/2688, and re-enact the majority of those provisions.
6.
Part 2 of these Regulations deals with the presentation and composition of feeding stuffs. The content of the statutory statement and other declarations are prescribed by regulation 8 and Schedule 3 and their form by regulation 9. (The labelling of additives and premixtures not mixed with feeding stuffs is now regulated directly by Regulation (EC) No. 1831/2003).
7.
The Regulations with minor drafting amendments also re-enact provisions of the 2000 Regulations so as to—
(a) prescribe the limits of inaccuracy permitted in the declaration of ingredients (regulation 10 and Schedule 4);
(b) attribute meanings to the names of certain materials for the purposes of section 70 of the Act (which creates an implicit warranty that material described by a name to which a meaning has been assigned under that section accords with the meaning (regulation 11);
(c) prescribe the way in which compound feeds may be sealed and packaged (regulation 12);
(d) regulate the putting into circulation and use of feed materials (regulation 13 and Schedule 2);
(e) restrict the putting into circulation or use of feeding stuffs containing specified undesirable substances (regulation 14 and Schedule 5);
(f) prohibit the putting into circulation or use of any feeding stuff containing certain prescribed substances (regulation 15);
(g) control the marketing and use of certain protein sources and non-protein nitrogenous compounds in feeds (regulation 16 and Schedule 6);
(h) regulate the iron content of milk replacer feeds (regulation 17);
(i) prohibit the putting into circulation of compound feeding stuffs in which the amount of ash insoluble in hydrochloric acid exceeds specified levels (regulation 18); and
(j) control the marketing of feeds intended for particular nutritional purposes (dietetics) (regulation 19 and Schedule 7).
8.
These Regulations provide for the implementation of Commission Directive 2004/116/EC mentioned above by including Candida guilliermondii among the substances authorised and regulated by regulation 16 and Schedule 6, and in regulation 20 provide for the execution and enforcement of the Additives Regulation by—
(a) making it an offence not to comply with certain specified requirements in the Additives Regulation (paragraphs (1) and (2));
(b) giving effect to the transitional arrangements in the Additives Regulation relating to products already on the market that were authorised under superseded EC legislation (paragraph (3)); and
(c) giving effect to the transitional arrangements in the Additives Regulation relating to applications for authorisation under the superseded EC legislation that were still being processed at the date of application of the Additives Regulation.
9.
In relation to feed additives these Regulations also maintain the duty of confidentiality imposed by the 2000 Regulations on anyone who may, in the course of processing an application for authorisation, have acquired commercially sensitive information (regulation 21).
10.
Part 3 of these Regulations deals with enforcement. It re-enacts provisions in the 2000 Regulations that—
(a) provide for the enforcement of requirements where the legal basis is the European Communities Act by linking such requirements to enforcement provisions in the Act (regulation 22);
(b) modify section 74A of the Act and provide for offences and penalties in relation to matters covered by the Regulations that would not otherwise come within that section (regulation 23); and
(c) amend, in relation to England, the Feeding Stuffs (Sampling and Analysis) Regulations 1999 in the same way as that expressed as a modification in the 2000 Regulations, and also make consequential amendments to the 1999 Regulations mentioned above (regulation 24).
11.
The EC Directives implemented by these Regulations are—
(a) Council Directive 70/524/EEC (OJ No. L270, 14.12.70, p. 1) concerning additives in feedingstuffs, (to the extent that its measures are preserved by Regulation (EC) No. 1831/2003), as last amended by Council Regulation (EC) No. 1756/2002 (OJ No. L265, 3.10.2002, p. 1);
(b) Council Directive 79/373/EEC (OJ No. L86, 6.4.79, p. 30) on the circulation of compound feedingstuffs, as last amended by Council Regulation (EC) No. 807/2003 (OJ No. L122, 16.5.2003, p. 36);
(c) Council Directive 82/471/EEC (OJ No. L213, 21.7.82, p. 8) concerning certain products used in animal nutrition, as last amended by Commission Directive 2004/116/EC (OJ No. L379, 24.12.2004, p. 81);
(d) Council Directive 93/74/EEC (OJ No. L237, 22.9.93, p. 23) on feedingstuffs intended for particular nutritional purposes, as last amended by Council Regulation (EC) No. 806/2003 (OJ No. L122, 16.5.2003, p. 1);
(e) Commission Directive 94/39/EC (OJ No. L207, 10.8.94, p. 20) establishing a list of intended uses of animal feedingstuffs for particular nutritional purposes;
(f) Council Directive 96/25/EC (OJ No. L125, 23.5.96, p. 35) on the circulation and use of feed materials, as last amended by Council Regulation (EC) No. 806/2003 (OJ No. L122, 16.5.2003, p. 1);
(g) Directive 2002/32/EC of the European Parliament and of the Council (OJ No. L140, 30.5.2002, p. 10) on undesirable substances in animal feed, as last amended by Commission Directive 2003/100/EC (OJ No. L285, 1.11.2003, p. 33); and
(h) Commission Decision 2004/217/EC adopting a list of materials whose circulation or use for animal nutrition purposes is prohibited (OJ No. L67, 5.3.2004, p. 31).
12.
A full regulatory impact assessment of the effect that this instrument will have on the costs of business has been prepared and placed in the Library of each House of Parliament together with a transposition note setting out how the provisions of Commission Directive 2004/116/EC have been transposed into domestic law by these Regulations. Copies may be obtained from the Primary Production Division of the Food Standards Agency, Aviation House, 125 Kingsway, London WC2B 6NH.
Notes:
[1]
1970 c. 40. Section 66(1) contains definitions of the expressions "the Ministers", "prescribed" and "regulations"; the definition of "the Ministers" was amended by the Transfer of Functions (Wales) (No. 1) Order 1978 (S.I. 1978/272), Schedule 5, paragraph 1. Functions of "the Ministers", so far as exercisable in relation to Wales, were transferred to the National Assembly for Wales by S.I. 1999/672. Those functions, so far as exercisable in relation to Scotland, were transferred to the Scottish Ministers by section 53 of the Scotland Act 1998 (1998 c. 46). By virtue of S.I. 1999/3141, functions of the Secretaries of State for Wales and Scotland previously exercisable in relation to England ceased to be so exercisable and were transferred to the Minister of Agriculture, Fisheries and Food. Section 74A was inserted by the European Communities Act 1972 (1972 c. 68), Schedule 4, paragraph 6.back
[2]
S.I. 2000/656.back
[3]
S.I. 2002/794.back
[4]
S.I. 1972/1811 in relation to the common agricultural policy and S.I. 1999/2027 in relation to measures in the veterinary and phytosanitary fields for the protection of public health.back
[5]
1972 C. 68.back
[6]
OJ No. L31, 1.2.2002, p. 1, as last amended by Regulation (EC) No. 1642/2003 of the European Parliament and of the Council (OJ No. L245, 29.9.2003, p. 4).back
[7]
OJ No. L270, 14.12.70, p. 1 (OJ/SE Vol. 18, p. 4) last amended by Council Directive 1999/20/EC (OJ No. L80, 25.3.1999, p. 20).back
[8]
OJ No. L268, 18.10.2003, p. 29. Last amended by Commission Regulation (EC) No 378/2005 (OJ No. L59, 5.3.2005, p. 8).back
[9]
OJ No. L155, 12.7.71, p. 13 (OJ/SE 1971(II), p. 480).back
[10]
OJ No. L213, 21.7.82, p. 8. Last amended by Commission Directive 2004/116/EC (OJ No. L379, 24.12.2004, p. 81).back
[11]
OJ No. L86, 6.4.79, p. 30. Last amended by Council Regulation (EC) No 807/2003 (OJ No. L122, 16.5.2003, p. 36).back
[12]
OJ No. L35, 8.2.2005, p. 1.back
[13]
OJ No. L279, 20.12.71, p. 7 (OJ/SE 1971(III), p. 987). (Part IV was replaced entirely by Annex 1 to Directive 84/4/EEC (OJ No. L15. 18.1.84, p. 28. That Annex was in turn replaced entirely by Part B of the Annex to Directive 98/64/EC (OJ No. L257, 19.9.98, p. 14)).back
[14]
OJ No. L92, 7.4.90, p. 42.back
[15]
OJ No. L125, 23.5.96, p. 35. Last amended by Council Regulation (EC) No. 806/2003 (OJ No. L122, 16.5.2003, p. 1).back
[16]
OJ No. L83, 30.3.73, p. 21. (Point 3 of Annex 1 was replaced entirely by the Annex to Directive 92/89/EEC) (OJ No. L344, 26.11.92, p. 35)).back
[17]
S.I. 2002/843, as amended by S.I. 2002/1253, S.I. 2002/2860, S.I. 2003/1482 and S.I. 2004/1518.back
[18]
OJ No. L279, 20.12.71, p. 7 (OJ/SE 1971(III), p. 987), amended by Article 1 of Directive 73/47/EEC (OJ No. L83, 30.3.73, p. 35).back
[19]
OJ No. L279, 20.12.71, p. 7 (OJ/SE 1971(III), p. 987). (Part IV was replaced entirely by Annex 1 to Directive 84/4/EEC (OJ No. L15. 18.1.84, p. 28). That Annex was in turn replaced entirely by Part B of the Annex to Directive 98/64/EC (OJ No. L257, 19.9.98, p. 14).back
[20]
S.I. 2002/843, as amended by S.I. 2002/1253, S.I. 2002/2860, S.I. 2003/1482 and S.I. 2004/1518.back
[21]
OJ No. L123, 29.5.72, p. 6 (OJ/SE 1966-1972 supplement, p. 74), (Point 2 of Annex 1 has been replaced by the Annex to Directive 93/28/EEC (OJ No. L179, 22.7.93, p. 8)).back
[22]
OJ No. L123, 29.5.72, p. 6 (OJ/SE 1966-1972 supplement, p. 74), (Point 1 of Annex 1 has been replaced entirely by the Annex to Directive 1999/79/EC (OJ No. L209, 7.8.1999, p. 23)).back
[23]
S.I. 2000/2481, as last amended by S.I. 2004/2688.back
[24]
OJ No. L268, 18.10.2003, p. 29.back
[25]
OJ No. L123, 24.4.98, p. 1, as amended by Regulation (EC) 1882/2003 of the European Parliament and of the Council (OJ No. L284, 31.10.2003, p. 1).back
[26]
OJ No. L135, 30.5.1991, p. 40, as last amended by Regulation (EC) 1882/2003 of the European Parliament and of the Council (OJ No. L284, 31.10.2003, p. 1).back
[27]
OJ No. L273, 10.10.2002, p. 1, as amended by Commission Regulation (EC) 808/2003 (OJ No. L117, 13.5.2003, p. 1).back
[28]
OJ No. L194, 25.7.95, p. 39, as last amended by Regulation (EC) No. 1882/2003 of the European Parliament and of the Council (OJ No. L284, 31.10.2003, p. 1).back
[29]
OJ No. L67, 5.3.2004, p. 31.back
[30]
OJ No. L151, 19.6.2003, p. 38, amending Directive 2002/32/EC of the European Parliament and of the Council on undesirable substances in animal feed (OJ No. L140, 30.5.2002, p. 10).back
[31]
S.I. 1999/1663. These Regulations are subject to a number of amendments but none is relevant.back
[32]
OJ No. L123, 29.5.72, p. 6 (OJ/SE 1966–1972 supplement, p. 74). Point 2 of Annex 1 has been replaced entirely by the Annex to Directive 93/28/EC (OJ No. L179, 22.7.93, p. 8).back
[33]
OJ No. L279, 20.12.71, p. 7 (OJ/SE 1971(III), p. 987). Part IV was entirely replaced by Annex 1 to Directive 84/4/EEC (OJ No. L15, 18.1.84, p. 28). That Annex was in turn replaced entirely by Part B of the Annex to Directive 98/64/EC (OJ No. L257, 19.9.98, p. 14).back
[34]
OJ No. L123, 29.5.72, p. 6 (OJ/SE 1966-1972 supplement, p. 74) (as replaced entirely by the Annex to Directive 1999/79/EC (OJ No. L209, 7.8.1999, p. 23)).back
[35]
OJ No. L155, 12.7.71, p. 13 (OJ/SE 1971(II), p. 480) as corrected by a corrigendum published in July 1975 (consolidated edition of corrigenda to the first series of specified editions of EC legislation (1952 to 1972)).back
[36]
OJ No. L155, 12.7.71, p. 13 (OJ/SE 1971(II), p. 480).back
[37]
Council Directive 90/167/EEC (OJ No. L92, 7.4.1990, p. 42) laying down the conditions governing the preparation, placing on the market and use of medicated feedingstuffs in the Community.back
[38]
OJ No. L206, 29.7.78, p. 43.back
[39]
OJ No. L273, 10.10.2002, p. 1.back
[40]
S.I. 2001/3389.back
[41]
S.I. 2002/892.back
[42]
S.I. 2003/1026.back
[43]
S.I. 2003/1503.back
[44]
S.I. 2003/2912.back
[45]
S.I. 2004/1301.back
[46]
S.I. 2004/2688.back
ISBN
0 11 073694 X
| © Crown copyright 2005 |
Prepared
5 December 2005
|
BAILII:
Copyright Policy |
Disclaimers |
Privacy Policy |
Feedback |
Donate to BAILII
URL: https://www.bailii.org/uk/legis/num_reg/2005/20053281.html



























































