Stem Cell Tourism: Assessing the State of Knowledge
Aaron D. Levine*
|
Cite as: A D Levine, "Stem Cell Tourism: Assessing the State of
Knowledge", (2010) 7:2 SCRIPTed 283,
http://www.law.ed.ac.uk/ahrc/script-ed/vol7-2/levine.asp
|
© Aaron D. Levine 2010.
This work is licensed under a Creative Commons Licence. Please click on the link to read the terms and conditions. |
1. Introduction
Stem cell science, using both embryonic and tissue-specific stem cells, is advancing at a rapid pace and offers substantial promise to improve the quality of life for a wide range of patients. Yet, for the most part, this promise is long-term and stem cells are primarily useful today as a basic research tool. Despite the preliminary nature of most stem cell research, a number of clinics around the world offer stem cell “therapies” to patients today.1 Although the popularity of these stem cell based interventions (SCBIs) is unknown, anecdotal reports suggest that a substantial number of patients are willing to try them, despite serious questions about their safety and efficacy.2 These patients hope to benefit from these experimental SCBIs. But this is, at best, a questionable assumption and these patients are essentially offering themselves as paying human subjects for unregulated, perhaps unjustified, and potentially dangerous research. They are, in essence, taking part in “a vast human experiment.”3
Patient pursuit of experimental interventions is not unique to stem cell science.4 Due to the politicisation of this field, however, scientists and policymakers are concerned that increased use of these interventions poses unacceptable risks, not just to individual patients, but also, more broadly, to the pursuit of legitimate stem cell research.5 These dual concerns were one motivation behind the decision of the International Society for Stem Cell Research (ISSCR) to issue Guidelines for the Clinical Translation of Stem Cell Research and to produce a Patient Handbook on Stem Cell Therapies.6 Similar policy concerns have also been voiced by other organisations, including government agencies, scientific associations and disease advocacy groups.
Travelling in pursuit of an experimental SCBI has come to be known as “stem cell tourism”. Driven largely by continuing policy interest, a growing interdisciplinary body of literature addresses this practice. This article reviews this literature to assess the state of knowledge of stem cell tourism and to identify important unanswered questions for future study. Understanding stem cell tourism is important for efforts that are designed to mitigate the risks it poses to both individual patients and the broader research field.
The article is organised as follows: the first section reviews the knowledge of the providers of unproven SCBIs; the second section focuses on the patients who travel in pursuit of unproven SCBIs; the third section reviews knowledge of the outcomes of these interventions and discusses the challenges of accurately assessing outcomes of these SCBIs; the article concludes by identifying important questions not yet, or only partially, addressed in the existing literature.
2. The Providers
Most clinics offering SCBIs to patients maintain a website to advertise their services. These websites have provided a source of relatively accessible information and, as a result, the providers of unproven SCBIs have been examined in both media reports and research articles.
A news article published in the journal Science in 2006, for instance, documented the existence of nine clinics offering unproven SCBIs.7 Four of these clinics claimed to use fetal cells of some sort, while three claimed to use umbilical cord blood stem cells, and two claimed to use autologous stem cells isolated from patients. The clinics were distributed around the world, with some operating in Europe and others in the former Soviet Union, China and the Caribbean. The article reported on one clinic, BioMark International, which had been operating out of the United States, but was shut down following an FDA investigation.8
Two recent studies have systematically examined the websites of clinics offering unproven SCBIs in an attempt to learn more about the practice of stem cell tourism. The first of these studies, published in late 2008 by Darren Lau and colleagues, identified nineteen clinics that advertised SCBIs directly to patients over the Internet,9 while the second study, published in May 2009 by Alan Regenberg and colleagues, identified twenty-three providers with accessible websites.10 Both studies collected data in mid-2007 and, thus, provide comparable insights into the providers offering stem cell based therapies at this point in time.
Both assessments provide information on the geography of stem cell tourism. The nineteen clinics examined by Lau et al offered SCBIs in fourteen different countries. Asian countries, including China, Thailand and the Philippines, accounted for the most treatment locations. Several organisations offered treatments in Central America or the Caribbean and a few locations were distributed among European countries.11 The twenty-three providers Regenberg et al examined were associated with thirty-seven different delivery locations. Just over half of the delivery locations were in Asia with most of the remainder divided between Europe and Central America/Caribbean.12 The fact that several providers offered treatment in multiple countries, or in multiple locations within the same country, accounts in part for the differences between the two studies. Neither study reported any providers that administered SCBIs to patients within the United States, although Regenberg et al noted that some providers maintained administrative offices in the United States from which they could refer potential patients to offshore clinics.13
Both of these studies also examined the types of stem cells that the providers claimed to use. These data are presented with the acknowledgement that they are self-reported by stem cell clinics and with the proviso that the nature of the cells actually used has not been independently verified. Each group identified providers that offered interventions with autologous adult stem cells, fetal stem cells, cord blood stem cells and embryonic stem cells. According to Regenberg et al’s analysis, nearly three fourths of the stem cell clinics offered only one type of stem cell, while the remaining clinics either offered a range of different cell types or treatments that included multiple cell types.14 Most clinics claimed to use human cells, but a handful were identified that offered xenotransplantations using sheep, shark or rabbit cells.15 Beyond these statements from the providers and the occasional comment from patients, little is known about the cells transplanted during these treatments and the possibility exists that some providers are not using stem cells at all. Characterising the cells used in these treatment attempts would be an important step toward understanding and evaluating unproven SCBIs.
Stem cell providers’ websites are essentially advertising tools designed to capture the attention of potential patients. Accordingly, attention has been dedicated to the advertising practices used by various providers and, more specifically, to the accuracy of the claims made by the clinics. Lau et al assessed providers’ websites to evaluate the extent to which the risks and benefits of the various SCBIs were discussed and the extent to which interventions were presented as routine, as opposed to experimental. They found a “general portrayal of therapy as safe and effective for a broad range of diseases in the context of routine clinical use.”16 A small number of providers were more circumspect, however, listing clear and bounded indications for treatments or offering a more tentative discussion of potential benefits. Lau et al also conducted literature searches to assess whether peer-reviewed literature existed that supported the routine use of the SCBIs reported on the clinics’ websites. They found that the SCBIs which these clinics offered generally lacked such support.17
Information about individual clinics can also be found in the mainstream media. Sometimes this information appears in news articles about specific patients and seems to simply repeat claims made by the clinic. In other cases, news organisations have conducted investigative reports into specific clinics. Assessing the accuracy of these media reports is beyond the scope of this article. Such an assessment remains an important task, however, particularly given the role that media reports may play in patient decision-making about SCBIs.
Although focused examinations of individual clinics have been rarer in the scholarly literature, a few have appeared in recent years. Beike Biotechnology, a Chinese company that claims its stem cells have been used to treat in excess of 5,000 patients using primarily umbilical cord blood stem cells and bone marrow stem cells,18 is among the providers that have been examined. As part of an analysis of translational research in China, Haidan Chen showed how Beike Biotechnology has pursued a controversial bedside-to-bench research model in which they jumped directly to clinical applications and hope this applied work will later inform basic research.19 Critics of Beike argue that the company is taking advantage of patients by charging large sums of money for treatments that have not been demonstrated to be safe or effective in clinical trials, while Beike representatives, according to Chen’s interviews, claim an obligation to provide treatments that “might bring patients some hope,” particularly when patients are suffering from incurable diseases.20 The Chinese government adopted new regulations in May 2009 designed to clamp down on the administration of unproven SCBIs and other experimental treatments.21 The extent to which these regulations will impact Beike’s operations remains an open question, although as of early 2010, the company still appeared to be actively recruiting patients.
Recent research by Patra and Sleeboom-Faulkner has examined unproven SCBIs in India and, in particular, explored patient recruitment through cases studies of two hospital groups in India.22 Although the hospital groups studied are not identified by name, the research highlights how the Indian regulatory approach, and the hub and spoke structure of the stem cell industry in India, facilitates recruitment of patients for unproven SCBIs.
These studies have identified both similarities and differences among the clinics offering unproven SCBIs. This heterogeneity suggests that patients and policymakers should not view all stem cell clinics as identical but rather consider the merits and demerits of each independently.23 Additional clinic-specific studies or comparative studies that move beyond clinic-supplied data would be valuable additions to the current literature base. Research on clinic-patient interactions during the recruitment and treatment processes would be particularly useful as differences in the information provided to potential patients about potential benefits and risks or supporting pre-clinical data could help outside clinicians evaluate the claims made by various clinics.
3. The Patients
Compared to the providers of unproven SCBIs, who advertise their services prominently on the Internet, relatively little is known about the patients who pursue these interventions. This dearth of knowledge is not surprising, given the dispersed nature of these patients and the understandable desire of many of them to maintain their privacy and not draw attention to their medical conditions or their use of unproven SCBIs. To my knowledge, only two published articles provide even partially systematic estimates of the characteristics of this population.
The first such study was Regenberg et al’s previously discussed content analysis of clinic websites.24 Eleven of the twenty-three clinics examined in this analysis presented case studies on their websites. In all, 533 different case studies were presented on eleven clinic websites. The patients described in these case studies were predominantly male (66%) and distributed among several age groups (<18: 10%, 18-39: 29%, 40-64: 50%, ≥65: 11%). The most common indications among these case studies were amyotrophic lateral sclerosis (34%), spinal cord injury (23%), multiple sclerosis (14%), heart disease (8%) and cerebral palsy (6%).25 These case studies provide useful insight into the patients pursuing unproven SCBIs but the extent to which they are representative of the larger population is unknown. It may be the case, for instance, that one or more of the clinics providing case studies focuses its SCBIs on a small number of indications, and, thus, skews these data. The possibility also exists that at least some of these case studies are fabrications, created and presented online solely as marketing tools.
The other attempt to characterise this patient population (of which I was a co-author) analysed the blogs of patients who had travelled internationally for an unproven SCBI.26 In all, this study analysed 161 blogs documenting the experiences of 162 patients who had undergone unproven SCBIs. The gender split (58% male) of these patients was similar to that reported in the analysis of case studies. The patients documented in the blog analysis were typically much younger, with 45% of those whose age could be determined under eighteen years old.27 This age distribution may reflect a greater comfort of younger patients with blogging as a form of communication. Alternatively, it may reflect the use of blogs as a public fundraising tool, which may be more common among parents raising money to help afford an unproven SCBI for a young child.
The most common indications among the patients described in these blogs partly overlapped with the indications reported in the analysis of the case studies. Spinal cord injury (20%), cerebral palsy (10%) and multiple sclerosis (10%), for instance, were among the most common indications in both studies.28 The second most common indication in the analysis of patient blogs was optic nerve hypoplasia, a congenital form of blindness that is typically diagnosed early in childhood development. This condition was not listed among the common indications in the analysis of case studies, suggesting these two approaches are capturing different subsets of this patient population.
Patient blogs offer some advantages and some disadvantages when compared with case studies posted on clinic websites for characterising this patient population. Many blogs are hosted on independent websites, making it less likely that they are controlled by the clinics. In addition, the blogs often provided a large number of details unrelated to the actual therapy; independent verification of some of these details provided additional evidence that the patients in the blogs were real rather than fabrications. The patients choosing to blog about their experiences with unproven SCBIs, however, may well not be representative of the larger patient population.
Blogs consist of a
series of dated entries. The time stamp associated with each entry
permits an assessment of when the 162 patients received their SCBIs.
The month of first SCBI received by each of the 162 patients is shown
in Figure 1.29
This chart shows a rapid increase over time in the number of patients
that blog about unproven SCBIs and these results are strongly
suggestive of an increase in the overall number of patients receiving
these interventions. Confirmation of this trend using more
representative data would be an important addition to the current
literature on stem cell tourism and help define the scope of the
risks posed by unproven SCBIs.
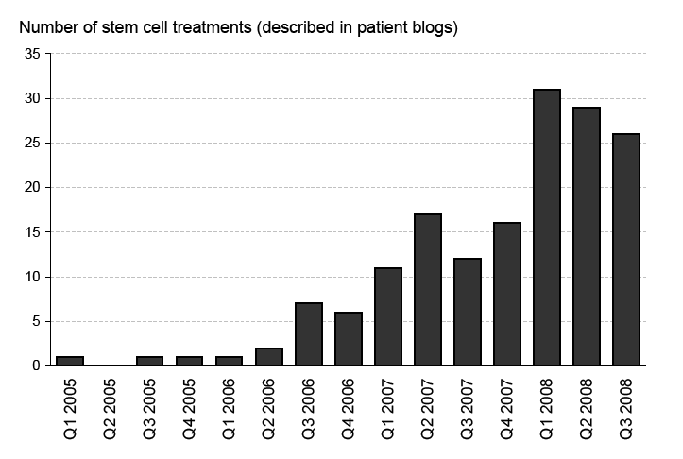
Figure 1 – Growth in patient blogs about unproven stem cell therapies30
Note: Based on the date of each patient’s first stem cell treatment
4. The Outcomes
Assessing the outcomes associated with unproven SCBIs is an important but challenging task. Like other patient self-reports, the blogs discussed above are not appropriate for analysing either safety or efficacy. Given the nature of these interventions, large placebo effects are both plausible and indeed likely. Moreover, patients who have relied on friends and family to finance their treatment may feel social pressure to report positive outcomes. Independent and objective case studies would offer some potential for evaluating the outcomes associated with unproven SCBIs. Existing case studies feature mainly subjective outcome measures,31 however, and were reported by clinics, which clearly have an interest in presenting positive outcomes.
Regulatory agencies, scientific societies and others have called for clinical trials, which would provide useful data on the outcomes associated with SCBIs. However, these calls have thus far gone unheeded. As a result, patients and clinicians have very little reliable data on which to assess these interventions, forcing them to turn to media coverage, patient self-reports and other sources not well suited for this task. Among the limited exceptions to this trend are two reports from a group of physicians that visited a Beijing clinic that has reportedly treated at least 400 spinal cord injury and 100 amyotrophic lateral sclerosis patients with cells extracted from fetal brain tissue and characterised by the clinic as fetal olfactory bulb-derived cells. A case study examining one spinal cord injury patient found a “rapid partial recovery of function” within a few days of surgery.32 The mechanism of the improvement was unknown but speculated causes included “increased functioning of intact fibres or strengthening of their synaptic connections.”33 The improvement was deemed unlikely to be due to regeneration or myelin repair. A second study examined seven spinal cord injury patients both before and after they went through a similar experimental SCBI, again using cells apparently derived from fetal brain tissue. In this case, systematic pre- and post-observations identified no clinically useful improvements and documented side effects, including meningitis, in five subjects.34 The authors concluded that in the absence of valid clinical trials, this intervention should not be recommended to patients.
In addition to these reports that focused on one SCBI provider in China, another peer-reviewed report has highlighted safety concerns associated with treatment by fetal neural stem cells at a hospital in Moscow. Specifically, this report documented the development of a donor-derived tumour in a patient with ataxia telangiectasia approximately four years after treatment with an experimental SCBI.35 This marked the first confirmed report of a tumour developing following an unproven SCBI.
5. Unanswered Questions
As the preceding discussion has illustrated, the literature on stem cell tourism is relatively recent and rather limited. Because their websites are easily accessible, a basic understanding of the providers of SCBIs has been developed. However, the extent to which these websites reflect the reality of treatment at these clinics as opposed to carefully calculated marketing messages remains an open question. Additional research focusing on specific clinics, such as the observational studies conducted in India,36 would be valuable. Although gaining access to these clinical sites would be challenging, first-hand observations of patient recruiting and treatment would provide important context to help evaluate existing studies of clinics’ websites and assess claims made by providers of unproven SCBIs.
Scholarly attention to others involved in stem cell tourism would also be warranted. To my knowledge, the opinions of patients who have received unproven SCBIs have not been systematically assessed and this would seem to offer substantial promise to yield useful information. Indeed, understanding how patients learned of these treatment options, how they decided to pursue them, and how they viewed their treatments would provide novel insight into the practice of stem cell tourism. Such research might also illuminate relevant differences between various providers. Research specifically focusing on patient decision-making might do well to include not just those individuals who decided to receive a SCBI, but also those who considered this option but ultimately rejected it. Evaluation of potential sources of information about stem cell tourism, such as newspaper articles or news broadcasts, would also be valuable.
It seems likely that many patients discuss unproven SCBIs with their physicians prior to pursuing these treatment options, yet doctors’ views of these interventions are not well understood. Understanding doctors’ awareness of these treatments and the advice they give their patients would be useful and might assist efforts by organisations, such as the ISSCR, to discourage patient pursuit of unproven SCBIs. Such data would also be relevant to ongoing discussions of the ethical obligations that physicians owe their patients (especially their minor patients) who are considering an unproven SCBI.37
Addressing the shortage of data on the outcomes of unproven SCBIs may be the most pressing challenge. Most clinics appear uninterested in conducting proper clinical trials. This decision is often couched in terms of an ethical obligation to offer treatment to patients immediately,38 but could also plausibly reflect a worry that an objective trial may prove their treatment approach ineffective and reduce demand for a profitable intervention. Some regulatory bodies may force providers to halt the administration of SCBIs pending the completion of clinical trials. However, given the relatively weak regulatory schemes in many countries where these interventions are administrated, this seems unlikely to be a common occurrence.39 Given this reality, the best option may be systematic observational studies conducted by disinterested third parties. Such studies should include objective pre- and post-measurements, preferably with long-term follow-up, and should not be administered by clinics or by others perceived to have a vested interest in the outcomes.
Stem cell tourism has emerged as a policy concern in the last few years. Already, a variety of studies from a range of academic disciplines have begun to explore the practice, yet many important questions remain unanswered. Continued scholarly attention to the growing practice of stem cell tourism is warranted. Such studies can only help ongoing efforts to mitigate the risks associated with unproven SCBIs and assure that translational stem cell research proceeds in an efficient and ethical manner.
* Assistant Professor, School of Public Policy, Georgia Institute of Technology.
1 D Lau et al, “Stem Cell Clinics Online: The Direct-to-Consumer Portrayal of Stem Cell Medicine” (2008) 3 Cell Stem Cell 591-594; A Regenberg et al, “Medicine on the Fringe: Stem Cell-Based Interventions in Advance of Evidence” (2009) 27 Stem Cells 2312-2319.
2 M Enserink, “Selling the Stem Cell Dream” (2006) 313 Science 160-163.
3 B Vestag, “Injections of Hope: Doctors Promote Offshore Stem Cell Shots, but Some Patients Cry Foul” (2 Sept 2008) Washington Post HE01.
4 On unproven HIV treatments see P Arno and K Feiden, Against the Odds: The Story of AIDS Drug Development, Politics, and Profits (New York: HarperCollins, 1992). On unproven cancer treatments see A Vickers, “Alternative Cancer Cures: ‘Unproven’ or ‘Disproven’?” (2004) 54 CA: A Cancer Journal for Clinicians 110-118.
5 I Hyun et al, “New ISSCR Guidelines Underscore Major Principles for Responsible Translational Stem Cell Research” (2008) 3 Cell Stem Cell 607-609; B Nelson, “Stem Cell Researchers Face Down Stem Cell Tourism” (2008) available at: http://www.nature.com/stemcells/2008/0806/080605/full/stemcells.2008.89.html (accessed 26 Apr 2010).
6 International Society for Stem Cell Research, “Guidelines for the Clinical Translation of Stem Cells” (2008) available at http://www.isscr.org/clinical_trans/pdfs/ISSCRGLClinicalTrans.pdf (accessed 14 Mar 2010); International Society for Stem Cell Research, “Patient Handbook on Stem Cell Therapies” (2008) available at http://www.isscr.org/clinical_trans/pdfs/ISSCRPatientHandbook.pdf (accessed 14 Mar 2010).
7 Enserink, see note 2 above.
8 A Zarembo, “A Desperate Injection of Stem Cells and Hope” (20 Feb 2005) Los Angeles Times A1.
9 Lau, see note 1 above.
10 Regenberg, see note 1 above.
11 Lau, see note 1 above.
12 Regenberg, see note 1 above.
13 Ibid.
14 Ibid.
15 Lau, see note 1 above.
16 Ibid.
17 Ibid.
18 D McMahon et al, “Cultivating Regenerative Medicine Innovation in China” (2010) 5 Regenerative Medicine 35-44.
19 H Chen, “Stem Cell Governance in China: From Bench to Beside?” (2009) 28 New Genetics and Society 267-282.
20 Ibid, 274.
21 D Cyranoski, “Stem-Cell Therapy Faces More Scrutiny in China” (2009) 459 Nature 146-147.
22 P Patra and M Sleeboom-Faulkner, “Bionetworking: Experimental Stem Cell Therapy and Patient Recruitment in India” (2009) 16 Anthropology & Medicine 147-163.
23 See, generally, C Murdoch and C Scott, “Stem Cell Tourism and the Power of Hope” (2010) 10 American Journal of Bioethics 16-23.
24 Regenberg, see note 1 above.
25 Ibid, 2316.
26 K Ryan et al, “Tracking the Rise of Stem Cell Tourism” (2010) 5 Regenerative Medicine 27-33.
27 Ibid, 30.
28 Ibid, 31.
29 Ibid, 30.
30 Reproduced from Regenerative Medicine (2010) 5(1), 27-33 with permission of Future Medicine Ltd.
31 Regenberg, see note 2 above.
32 J Guest, L Herrera and T Qian, “Rapid Recovery of Segmental Neurological Function in a Tetraplegic Patient Transplantation of Fetal Olfactory Bulb-Derived Cells” (2006) 44 Spinal Cord 135-142.
33 Ibid, 139-140.
34 B Dobkin, A Curt and J Guest, “Cellular Transplants in China: Observational Study from the Largest Human Experiment in Chronic Spinal Cord Injury” (2006) 20 Neurorehabilitation and Neural Repair 5-13.
35 N Amariglio et al, “Donor-Derived Brain Tumor Following Neural Stem Cell Transplantation in an Ataxia Telangiectasia Patient” (2009) 6 PLoS Medicine e1000029.
36 Patra, see note 22 above.
37 A Zarzeczny and T Caulfield, “Stem Cell Tourism & Doctors’ Duties to Minors – A View from Canada” (2010) 10 American Journal of Bioethics 3-15.
38 Chen, see note 19 above.
39 See B von Tigerstrom, “Product Regulation and the Clinical Translation of Stem Cell Research” (2009) 5 Stem Cell Reviews 135-139 for a discussion of the regulatory challenges.


