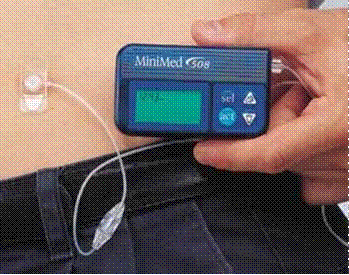
Covid-19 Protocol: This judgment was handed down by the judge remotely by circulation to the parties’ representatives by email and release to Bailii. The date and time for hand-down is deemed to be 10am on Friday, 9 July 2021.
Deputy Judge Treacy:
Introduction. 3
Conduct of the trial 3
The issues. 4
The witnesses. 4
The skilled team.. 6
The common general knowledge. 7
Agreed common general knowledge. 8
Disputed common general knowledge. 17
The Patent 23
Overview.. 23
Summary of the Invention. 24
Claims in Issue. 26
Legal principles - construction. 28
Claim interpretation. 30
Infringement 48
The Solo Kit, Solo Components and Solo System.. 48
The allegations of infringement 50
Claim 1. 53
Claim 2. 69
Claim 3. 70
Amended Claim 43. 71
Amended Claim 45. 71
Validity - Introduction. 72
Novelty/anticipation - the main legal principles. 72
Obviousness/inventive step - the main legal principles. 74
The prior art 75
PhiScience - Novelty. 79
PhiScience obviousness/inventive step. 90
MiniMed - obviousness / inventive step. 100
Added matter 104
Legal principles. 105
The allegations of added matter 105
Amendment 113
Appendix A 113
Appendix B 113
Introduction
1. The Claimant (“Insulet”) is the proprietor of EP (UK) 1 335 764 (the “Patent”) and is a manufacturer and supplier of insulin pumps.
2. The Patent is entitled “Device and system for patient infusion” and states that it relates particularly to “small, low cost, portable infusion devices that are useable to achieve precise, sophisticated, and programmable flow patterns for the delivery of therapeutic liquids to a mammalian patient”. It relates, among other things, to ambulatory insulin pumps, has a priority date of 8 September 2000 and expires on 30 August 2021.
3. Insulet has applied to amend the claims of the Patent unconditionally. A copy of the proposed amendment in full is attached as Appendix A for ease of reference. The issues are to be decided by reference to these unconditionally amended claims. The amendments are opposed on grounds of added matter.
4. Insulet relies on five claims as independently valid and infringed: Claims 38 and 40 of the Patent as granted and Claims 1, 42 and 44 as amended. Claims 38 and 40 have been renumbered Claims 2 and 3. The parties agreed to use the renumbered format (1, 2, 3, 43, 45). Throughout this Judgment these are referred to as the Claims in Issue.
5. The Defendant is Roche Diabetes Care (“Roche”). In mid-2018, Roche launched a line of insulin pumps which are referred to below as “Solo” products.
6. Insulet claims that Roche has infringed the Patent:
(i) directly, by the manufacture and sale of kits containing the Solo pump and related components; and/or
(ii) indirectly, by the supply of consumable components (such as replacement pumps, reservoirs, and remote controllers - “Solo Consumables”).
7. Roche denies infringement and counterclaims for revocation of the Patent, relying on two prior art documents, known as “PhiScience” and “MiniMed”, and on added matter.
8. Andrew Waugh QC appeared for Insulet, with Jaani Riordan. Michael Tappin QC appeared for Roche, with James Whyte. Mr Waugh QC undertook most of the oral advocacy for Insulet. Mr Riordan addressed the court on added matter.
Conduct of the trial
9. The trial was conducted fully remotely. This worked well, despite some minor technical glitches. Owing to the pandemic, Mr Causey, Insulet’s expert, gave evidence from California. To accommodate this, the Court sat for a little longer than the normal court day. As the proceedings were under PD57AB and formed part of the Shorter Trial Scheme, it also became necessary to adjust the court day a little on the final day of the trial to allow time to complete and submit written closings and to deliver oral closings.
10. I am grateful to the court staff and the shorthand writers for helping with these more flexible hours. I am also grateful to the parties’ solicitors for their efficient and helpful provision and updating of electronic bundles. In view of the compressed time available for the trial, the overnight transcript service was very useful.
11. Although the trial was fitted within the time allocated, it was necessary for all involved to maintain a high degree of discipline to achieve that result. The complexity of the issues and the number of areas of dispute raised during the cross-examination of the experts, and which then needed to be resolved, tested the outer limits of the time and procedures available under the Shorter Trial Scheme and have resulted in a longer post trial period than might have been the case had there been more time available during the hearing. I am grateful to counsel and to the expert witnesses for adapting accordingly during the hearing.
The issues
12. The issues for trial were set out in the PTR order:
· Does Claim 2 lack novelty over PhiScience;
· Does any of the Claims in Issue lack inventive step over PhiScience and/or MiniMed;
· Is any of the Claims in Issue invalid by reason of added matter;
· Is the Claimant permitted to amend EP ‘764 (the Patent);
· Does the Solo fall within the scope of the Claims in Issue;
· Do the Solo and/or Solo Consumables (together or individually) constitute means relating to an essential element of the invention of any of the Claims in Issue;
· Does the Defendant supply the Solo and/or Solo Consumables (together or individually) knowing, or where it is obvious to a reasonable person in the circumstances, that these are means suitable for putting and are intended to put the invention as claimed in the Claims in Issue into effect in the United Kingdom?
The witnesses
13. Each party relied on a single expert.
14. Insulet called Mr James Causey. At the priority date Mr Causey was VP of R&D at MiniMed. He was involved in new designs for insulin delivery systems and in developing MiniMed pumps. He is one of the named inventors of the MiniMed prior art. Mr Causey is clearly very knowledgeable about insulin delivery systems and about the industry around the turn of the last century, having been tasked as part of his role at MiniMed with researching insulin pumps offered by existing market participants including Disetronic, Deltec and others. He has subsequently maintained his interest in insulin delivery and pump systems.
15. I found Mr Causey’s evidence to be very helpful on many technical aspects of the dispute. Roche submitted that his experience in the industry meant that on certain issues he took an unduly narrow approach to the skilled team’s focus and likely approach, which was affected by the particular pre-occupations of the team with which he was working at MiniMed at the priority date. These were not criticisms of Mr Causey personally and are best dealt with where they arise below. In closing submissions, counsel for Roche also criticised some specific aspects of Mr Causey’s evidence. These are also dealt with where relevant below and do not require any wholesale review of the value of Mr Causey’s evidence to the Court.
16. Roche called Mr William Treneman, who is a mechanical engineer with broad experience in designing complex products including medical devices. Mr Treneman has no specific experience with insulin delivery systems or pumps. Among other things, Mr Treneman has worked on drug delivery devices including inhalers, intensive care intravenous pumps and subcutaneous auto-injectors. He has been involved in the provision of product development services for an array of high tech devices. Mr Treneman was clear in his written reports and in his oral testimony that he had prepared his evidence on the basis of written materials which he had researched (with some assistance from colleagues), bringing to bear his more general experience as an engineer in fields including medical devices (though not insulin pumps).
17. As with Mr Causey, I found Mr Treneman’s evidence to be clear and helpful on many of the issues. Insulet submitted that Mr Treneman’s lack of experience in the specific field of ambulatory infusion pumps undermined the value of the evidence he could give and criticised his approach, which was described as pedantic and unrealistic. Mr Treneman was particularly criticised for relying on documentary research into the insulin pump field and for being unable to speak from personal experience of any generally held ‘industry mindset’ at the priority date. Given the parties’ general agreement as to the nature of the ‘skilled person’ or ‘skilled team’ and the approach that team would have taken (see below), I do not consider Mr Treneman to be an unsuitable expert witness in this case.
18. Insulet further criticised the approach which Mr Treneman took to the prior art and the common general knowledge as being tainted by hindsight and criticised aspects of the sequencing of how he was introduced to the prior art and the Patent. While this may have been less than ideal, I note, as recently discussed by Meade J in Fisher & Paykel Healthcare Ltd v Flexicare Medical Lt & Anor [2020] EWHC 3282 (Pat), that the main risk to be avoided through sequencing is the risk of hindsight and that problems can be avoided as long as the expert reflects carefully on how their knowledge of the invention may influence them, and is disciplined in avoiding hindsight. Having heard Mr Treneman’s cross‑examination and reviewed his written evidence, I do not consider his evidence to have been substantially tainted by hindsight. On the one or two occasions where that was the case, I have made reference to it below and have adjusted my approach to his evidence accordingly, assessing the reasons for his conclusion in the light of that concern.
19. Mr Treneman also faced robust, sometimes very robust, questioning during cross‑examination and was described by Mr Waugh QC in closing submissions on behalf of Insulet as argumentative. The reasons for this comment perhaps lie in part in the short time available for cross-examination owing to the tight timetable inherent in trials under the Shorter Trial Scheme which placed significant burdens both on counsel and on the witnesses. On occasion, Mr Waugh QC felt it necessary to truncate some of Mr Treneman’s responses or moved forward with his questioning before Mr Treneman had located the relevant materials in his electronic bundle. This led to some frustration on the part of Mr Treneman. Nevertheless, I felt that Mr Treneman sought to explain his views to the Court throughout his oral testimony and that his overall approach during cross-examination primarily reflected his desire to communicate his views effectively.
20. In closing submissions, counsel for Insulet urged that I should reject the evidence of Mr Treneman and accept that of Mr Causey on all the issues where there was conflict. I do not regard it as appropriate to adopt such a wholesale approach in this instance. As with Mr Causey, Mr Treneman approached his evidence from his own perspective. As with Mr Causey, I deal with specific criticisms as far as is relevant below.
21. Given the comments made about both experts (for different reasons), I recall the helpful comments of Jacob LJ in Rockwater Ltd v Technip France SA & Anor [2004] EWCA Civ 381 at [12] and [15], that:
“I must explain why I think the attempt to approximate real people to the notional man is not helpful. It is to do with the function of expert witnesses in patent actions. Their primary function is to educate the court in the technology - they come as teachers, as makers of the mantle for the court to don. For that purpose it does not matter whether they do or do not approximate to the skilled man. What matters is how good they are at explaining things.” [12]
“Because the expert’s conclusion (e.g. obvious or not), as such, although admissible, is of little value it does not really matter what the actual attributes of the real expert witness are. What matters are the reasons for his or her opinion. And those reasons do not depend on how closely the expert approximates to the skilled man.” [15]
22. Both experts sought to discharge their duties to the Court. As pointed out by counsel for both parties (although not about the witness instructed by their respective clients), on occasions both experts showed an unwillingness to let go of positions they had adopted, or a tendency to adopt positions that were a little ill-advised. I deal as required with those issues below and do not regard those criticisms as sufficient to significantly undermine the overall evidence given by either expert.
23. While they came from quite different backgrounds, I found the evidence of both experts to be helpful and to contribute significantly to my understanding of the technical background to the dispute, of the state of the art and of the issues that faced the skilled person or skilled team at the priority date. Both of them generally gave clear reasons for the views that they held and it was helpful to appreciate some of the ways in which their specific experiences might affect their individual views. I did not find the differences in their personal experiences to be unhelpful and, indeed, having different perspectives was both interesting and useful, although it was also necessary to bear it in mind when assessing the evidence given. I found them both to be good educators.
The skilled team
25. The parties agreed that the Patent is addressed to a team working on the design of insulin pumps. The parties also agreed that the team would have been led by a medical device engineer, supported by other engineers, and with the benefit of input from clinicians.
26. It was common ground between the experts that the engineer member of the skilled team would have had a university degree in a field such as engineering or perhaps biological sciences and experience in designing medical devices. Mr Causey suggested that ideally the lead engineer would have had experience designing insulin delivery devices, or software-controlled drug delivery devices, but I did not understand him to say that this was a pre-requisite.
27. There were nuanced differences between the experts as to the approach that the skilled team would have taken. Both agreed that those tasked with designing an insulin pump would have familiarised themselves with the insulin pump field, including the insulin pumps that were on the market at the time such as the MiniMed and Disetronic pumps.
The common general knowledge
29. The requirements for Common General Knowledge (“CGK”) were summarised by Arnold J in KCI Licensing Inc & Ors v Smith & Nephew Plc & Ors [2010] EWHC 1487 (Pat) at [105]-[115] as that which is generally known to, and generally regarded as a good basis for further action by, those who are engaged in the particular art. Account may also be taken of information that, while it was not part of the skilled person’s common general knowledge, would have been acquired as a matter of routine before embarking on the problem to which the patent provides a solution: for example, information obtained from literature that it would have been obvious to review, or from routine testing that it would have been obvious to carry out.
30. Mr Waugh QC drew out in his skeleton argument three important attributes of CGK which, for convenience, I summarise below:
· The skilled addressee “… may not have the advantages that some employees of large companies may have”.
· The CGK consists of knowledge which forms “part of the mental equipment necessary for competency in [the] art or science concerned, such as every worker in the art may be expected to have as part of his technical equipment.”
· The CGK is such knowledge as would form “part of the stock of knowledge which will inform and guide the skilled person’s approach to the problem from the outset” and for this reason will affect “the steps it will be obvious for him to take, including the nature and extent of any literature search” (Generics (UK) Ltd v Daiichi Pharmaceutical Co Ltd [2009] EWCA Civ 646 at [26] (approving the approach of the trial judge, Kitchin J)). In other words, it must be asked whether “the skilled person faced with the problem to which the patent is addressed would acquire that information as a matter of routine”.
Agreed common general knowledge
31. Much was agreed by the parties to be CGK, although at the outset of the hearing there was no convenient summary of what was common ground. I agree wholeheartedly with the comments of Meade J in Fisher & Paykel (at [51] and following) that, in cases where there is no primer or textbook to which the Court can be referred, such a document is very valuable and the parties should in future consider providing such a document if no other convenient summary is available to assist the Court in pre-reading or in assessing the oral evidence. That is particularly the case in the cases under PD 57AB where the time for evidence is constrained and where the time for writing judgments is equally expected to be relatively short.
32. At my request, the parties submitted a document setting out those aspects of CGK on which they agreed, which I have reviewed and which forms the basis of the paragraphs which follow. The document saved considerable time in preparing this judgment, although a few issues that should perhaps have been agreed were left unresolved and it would have been more useful to have had the document sooner, particularly to assist in assessing the oral evidence.
Diabetes and the role of insulin
33. Patients suffering from diabetes mellitus experience dysglycaemia when the concentration of glucose in the blood stream moves above or below normal levels. If hyperglycaemia (high blood glucose) is left untreated it can cause serious complications including diabetic comas and damage to internal organs, the nervous system and the circulatory system. Symptoms of hyperglycaemia typically include thirst, weakness or tiredness, increased urination, blurred vision and, over time, loss of weight. Injury or death may result from sustained hyperglycaemia. Similarly, if hypoglycaemia (low blood glucose) is sustained, severe injury, coma or death may be the outcome.
34. Two forms of diabetes were recognised in western countries: insulin dependent diabetes mellitus (type 1 diabetes) and non-insulin dependent diabetes mellitus (type 2 diabetes). Type 1 diabetes is the more prevalent form among children and young adults and is sometimes referred to as “juvenile diabetes”, but it can develop in patients of any age. Type 1 diabetes is commonly diagnosed between the ages of 10 and 16 and affects about 10% of those diagnosed with diabetes.
35. Insulin is a hormone produced in the pancreas. In someone who is not suffering from diabetes, the pancreas detects the rising glucose concentration of the blood (e.g. following the breakdown of carbohydrates during the digestion of food) and insulin is secreted from the pancreas into the body. Glucose present in the blood stream is then taken up into the cells and is available for consumption during normal metabolic processes or for storage as fat. This causes the blood glucose concentration to fall again and once it reaches a threshold the pancreas will stop releasing insulin. In this way the pancreas of a healthy person can control blood glucose levels.
Type 1 diabetes and its treatment
36. In a person with type 1 diabetes, the pancreas is unable to produce insulin when blood glucose concentrations rise. Without endogenous insulin in the bloodstream, glucose concentration will remain high, depriving cells of the necessary materials to produce energy via normal metabolic processes which use glucose as fuel. Left untreated, glucose rises to toxic levels, resulting in hyperglycaemia.
37. A proportion of type 1 diabetics can experience poor visual and auditory functions from vascular and neurological damage, and stiffness in the hands which leads to poor hand strength and limited joint mobility and cardiovascular disease. Treatment methods for patients with type 1 diabetes must take account of these conditions as they can affect the patient’s ability to receive and understand instructions and operate medical devices.
38. Type 1 diabetes is normally a life-long condition. It is managed using a combination of exogenous insulin, dietary regulation, and exercise. Insulin was first made commercially available in 1923 in the US. Techniques for manufacturing human insulin were developed in the 1960s and 1970s, and by 2000 most insulin consumed globally was synthetic human insulin or human analogs.
Glucose measurement and dose calculation
39. Type 1 diabetic patients must regularly test the glucose concentration of their blood. This was typically done using finger pricks and electrochemical test strips, processed using a device called a glucose meter, or “glucometer”. Once a patient has measured their blood glucose concentration, they can calculate how much insulin would be required to return their glucose level to a target range (if too high) or how much food to ingest (if too low). Insulin bolus doses can be administered in anticipation of food that is about to be ingested, to counter the subsequent increase in blood glucose. Type 1 diabetic patients (or their parents, for young patients) must make a constant series of calculations across the day and learn to accurately estimate carbohydrates and fats in food.
Administering insulin
40. Insulin is typically administered subcutaneously. If a patient is hospitalised, insulin is administered either intravenously or subcutaneously by injection.
41. Insulin is provided in a range of concentrations measured in units/ml. The standard concentration is 100units/ml or U-100, but it can be sold at lower concentrations such as 50units/ml (U-50) or higher concentrations, such as 300units/ml (U-300).
42. The traditional method for subcutaneous provision of insulin was by filling a reusable syringe from a vial of insulin and self-injecting it into the patient multiple times daily, sterilising the syringe between use. The first disposable glass and plastic syringes for insulin were developed in the 1950s. They were widely used by the mid-1960s. These syringes were used in combination with vials of insulin.
43. To manage blood glucose levels well, a type 1 patient will require 2-4 injections, or more, a day. This may include a combination of different insulins.
44. Injector pens follow the same principles as the syringe/vial and typically comprise:
· an insulin container. This can be either a replaceable insulin cartridge (in a reusable pen) or an inbuilt prefilled cartridge (in a disposable pen). Different pens can have different sized containers.
· a needle holder and disposable needles. The needle is inserted when the patient is ready to administer a dose and removed and disposed of after use. There are a range of gauges of needle available.
· some form of dial to set the amount of insulin to be administered, often with intervals of half a unit or one unit of insulin.
· a window or display showing how much insulin is remaining in the container.
45. Injector pens require multiple doses across the course of day. They are generally considered to have advantages over syringe and vial methods including convenience, ease of use, accuracy and lower pain associated with an injection.
46. The first injector pen for diabetes treatment was launched in 1985 by Novo Nordisk. The first metal re-useable pens contained roughly one week’s supply of insulin in a glass cartridge. The dose could be varied by rotating a graduated knob on the pen top before injection. This allowed users to administer a bolus dose of rapid insulin of a specific, variable amount. During the 1990s, the majority of insulin pens were supplied pre-filled and were thus disposable, providing sufficient insulin for roughly one week. By late 2000, a number of different insulin injector pens were available in the UK.
47. Syringe or pen treatments involve Multiple Daily Injections of doses of insulin (MDII or MDI). MDII involves a variable number of injections, the timing and frequency of which will be adjusted according to the needs of the patient.
48. Implantable insulin pumps were rare in 2000 and were considered a separate class of treatment to Continuous Subcutaneous Insulin Infusion (“CSII”) therapy (see below). They comprise a pump unit which is surgically implanted into the peritoneal cavity, with an infusion cannula that delivers insulin into the peritoneal space. The device contains a reservoir of insulin which is filled via a needle inserted through the skin around once a month. The device is controlled using a handheld wireless communicator and delivers insulin from the reservoir as instructed.
49. Unlike syringe or pen treatments, (non‑implantable) insulin pumps operate on the basis of CSII. The ideas behind such pumps arose in the 1960s and 70s. Devices at that time were large, heavy, often unreliable and almost exclusively reserved for hospital or clinical settings. Dean Kaman is credited as the inventor of the first wearable (not body attached) insulin pump, the “AutoSyringe” in 1976. During the 1980s, smaller, more user-friendly commercial insulin pumps were made available to patients.
50. At their most basic, insulin pumps included some form of subcutaneous delivery mechanism which was left in place within a patient for an extended period (e.g. a needle or an infusion cannula), some form of insulin reservoir, and some means of driving the insulin from the reservoir into the patient via the delivery mechanism in a controlled manner. Commercially available CSII pumps typically comprised:
· a sealed reservoir to contain insulin, typically with a maximum volume of 3ml;
· a pump, with either a solenoid and wheel drive mechanism (in the case of MiniMed devices) or a leadscrew (later telescoping) drive (in the case of Disetronic devices);
· a connection between the reservoir and external tubing, typically using a threaded Luer connector to attach the tubing to the outlet of the reservoir;
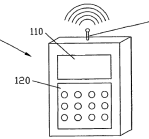
· external tubing. All CSII pumps on the market used external tubing, typically over 60cm in length. The tube connected at one end to the reservoir of the pump, and at the other end to the cannula at the penetration site;
· an infusion set consisting of a needle or cannula; typically a steel cannula or a soft cannula;
· operating interfaces on the pump housing, including buttons to control the pump and change the delivery settings, and a Liquid Crystal Display (“LCD”) screen.
51. Smaller insulin pumps were typically worn in pockets, or using belt clips or bra clips.
52. The infusion set was detached from the patient’s skin and the pump and replaced every few days. The site of infusion becomes resistant to the exogenous insulin at about three days. The labelling of insulin includes limitations requiring the infusion site to be changed at least at 3 day intervals.
53. Where an infusion set consisting of a cannula was used, it could be self-inserted using an auto-insertion tool.
54. A number of problems with tubing were well known at the priority date. Sometimes the infusion set would be dislodged or start leaking at the infusion site. Bubbles may enter the tubing and the pump would need to be primed before use, resulting in fluid loss. Tubing could catch on objects, and it could become occluded or detached.
55. The first MiniMed insulin pump was released in 1983 and was small enough to fit in the hand. In around 1998, MiniMed introduced a quick disconnect fitting which enabled the user to detach the tubing (and pump) from the cannula at the infusion site for a short period (for example, to enable the user to shower or swim).
56. The skilled person designing an insulin infusion pump would use standard tubing infusion sets available from specialist third party suppliers.
57. CSII pumps had a casing or case works allowing access to the device for the user to change the battery and fill the reservoir when needed.
58. With a pump and continuous insulin delivery, it is possible to deliver a small amount of insulin constantly to the patient, as well as to occasionally send a single larger volume of insulin down the tubing. This gives the option of setting two types of insulin flow:
· basal insulin (‘background’ insulin) is released constantly whether or not a patient is eating to regulate glucose levels between meals. It causes a consistent low-level flow from the reservoir of insulin into the patient; and
· bolus insulin is administered in response to the planned consumption of food and corrects blood glucose levels for the amount of food consumed. It presents as a short-term higher volume of insulin which is delivered from the reservoir into the patient after which the flow from the pump reduces back down to basal levels. The type of bolus delivered can depend on the length of the patient’s meal and on the glycaemic index of the food consumed.
59. When CSII through commercial insulin pumps first became available, it was not known whether it had any significant clinical advantages over MDI for the majority of patients. Without such advantages, it was unlikely that healthcare providers and payers would offer insulin pumps to a wide range of patients given the significantly higher costs compared with MDI. In 1993, the landmark Diabetes Control and Complications Trial (“DCCT”), which compared traditional MDI to both CSII and intensive insulin therapy, confirmed that CSII was superior to conventional MDI at controlling blood glucose, preventing short term complications such as hypoglycaemia and reducing the risk of long-term microvascular and neurological complications. The willingness of healthcare services and payers to cover insulin pump treatment started to rise after these clinical findings, albeit relatively slowly, requiring considerable effort from pump manufacturers.
60. Insulin pumps are regulated medical devices and in 2000 new insulin pumps were approved for sale by the CE Mark system in the UK and Europe, by the FDA in the USA, and by other national regulators. The FDA operated a process known as “510(K)” approval, which allowed a manufacturer to obtain approval by showing substantial equivalence to an existing authorised pump.
61. Insulin pumps tended to be used by a small proportion of type 1 diabetic patients. In the UK, the NHS was not routinely funding insulin pumps for patients so the figure was close to zero and almost all patients used syringes or pens.
62. Insulin pumps are used by individuals, including children and physically impaired patients, in their normal environment and not under the continuous direct supervision of a healthcare professional nor in a hospital or clinic.
Commercially available CSII pumps
64. The MiniMed 508 pump, shown below, was the leading ambulatory insulin pump in 2000, along with the Disetronic H-TRONplus. It had four buttons and an LCD screen on the device itself and had an IPX7 waterproof rating.
Figure 1: The MiniMed 508 pump
Figure 2: The MiniMed 508 pump and tubing 65. A table of the pre-priority devices which would have been known to the skilled person is shown below:
|
Model (year) |
Size and weight |
Remote control |
Key features |
|
MiniMed 502 (1983)

|
- |
None |
LCD screen |
|
MiniMed 506 (1992)

|
5.1 x 8.6 x 2cm
110g |
None |
Low battery and occlusion alarm
Larger screen Temporary basal rates
Safety checks (1440/day) 4 year warranty |
|
MiniMed 507 (1996)
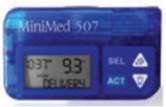
|
5.0 x 8.67
x 2cm 100g |
None |
Backlit screen Square wave boluses
Audio programming of boluses
Improved safety checks (15,000/day) 4 year warranty |
|
Disetronic H-TRONPlus (1997)

|
8.5 x 5.4 x 1.9cm 100g |
None |
24 programmable basal rates
Beeps to confirm delivery amount
Recessed top bolus buttons IPX7 waterproof
4 year warranty |
|
MiniMed 507C (1999)

|
5.0 x 8.67
x 2cm 100g |
None |
Could connect to PC to transfer pump data Improved record keeping Improved safety checks (30,000/hour)
Dual wave boluses 4 year warranty |
|
MiniMed 508 (1999)
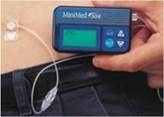
|
4.8 x 8.6 x 2cm
100g |
Optional keyring remote, with three buttons and no screen |
Remote programmer Vibration mode
Low reservoir volume alert Improved safety checks (40,000/hour)
4 year warranty |
67. The Disetronic H-TRONplus had three buttons, known as the S, h and m buttons. In run mode, the h and m buttons were used to program and deliver a bolus, and the S button was used for all other functions. It had an IPX7 waterproof rating.
68. The MiniMed 508 was the most recent MiniMed device at the priority date. It introduced a key fob style remote control device, which replicated a subset of the controls from the pump housing, with buttons for bolus delivery and on/off. It allowed setting of the bolus amount (in terms of the number of pre-programmed increments) but not setting of the basal rate:
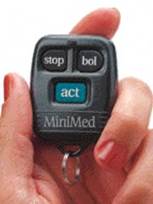
69. Other developments in CSII pumps involved improvements to the internal software (for example, better safety protocols, more basal rate profiles, and larger memory capacity for storage of insulin delivery data). Overall, progress in CSII pump development in the 1990s was incremental.
Buttons and switches
70. User input could be received via mechanical buttons, electromechanical switches or buttons, and other user interfaces such as touch screens.
71. The processor and electronic components would be connected by a printed circuit board. This would include a display strip connector which would typically be connected to an LCD display. Buttons such as membrane keypads were typically moulded in silicone rubber with a 2 to 3 mm diameter carbon pill in the middle of the button that contacted painted tracks on the printed circuit board when pushed inwards, forming an electrical contact. To prevent the ingress of water into the device via the button surround, the buttons could be sealed against the interior of the device case.
72. Electromechanical switches, normally printed circuit board mounted, could also be used. Such switches have a mechanical element that, when pressed, completes a circuit by touching electrical contacts on the other side of the button or substrate, thereby activating it.
Designing an insulin pump at the priority date
74. As the design and manufacture of insulin pumps was a small field, manufacturers would have been aware of competitors’ products and the features of competitor devices.
75. Companies which had pumps on the market would build on their existing technology. Patients typically use a single pump system for years, and may have more confidence in new pumps which are similar to previous models they have used, and that require less training. Given the potentially serious consequences of mismanaged insulin levels, familiarity and accuracy of operation are crucial and safety is a priority. The approach to design of those working in the field was quite conservative, focusing on incremental change and improvement and the highest levels of product quality and reliability.
76. The skilled person would have considered ways to improve the general functioning of the insulin pump, particularly as to the key design considerations set out below; and any new features of the pump that appeared to be advantageous.
77. Key considerations in pump design which would have been in the mind of a skilled person would have included:
· Accuracy: administering an excessive quantity of insulin or not delivering the required quantity at all could be dangerous and even fatal. Pumps would need to deliver the requested volume of insulin accurately (e.g. Target Dose + 5%).
· Reliability: pumps must be reliable and not prone to errors such as mechanical failures, failures in the pump software or incorrect use due to human factors. Key issues can include blockages/occlusions and the infusion set disconnecting from the pump. There would need to be a very low risk of accidentally triggering or cancelling an insulin delivery.
· Durability: to be cost effective pumps must usually be used for at least 4 years. The pump would need to be of sufficiently high quality so as to be unlikely to break or malfunction in that time.
· Portability: the pumps would need to be small and light enough to be easily carried and ideally, small enough to be discreet.
· User-friendliness / human factors: pump instrumentation would need to be user-friendly for the average diabetic patient, with intuitive displays and controls that the patient can learn to operate and helpful features that improve the process of managing a patient’s condition. Safe operation of the pump’s controls when the cognitive ability of a type 1 patient is impaired by dysglycemia is a major design consideration. Digital displays with clear, high contrast character fonts were very important.
· Regulatory approval: insulin pumps and infusion sets required approval from the local national or regional regulatory agency e.g. the FDA in USA and the CE marking system, through a Notified Body, in the EU. The design team would have been conscious of the likelihood and speed of gaining approval.
· Cost: a CSII pump typically cost $4,000-$6,000. Any insulin treatment that cost significantly more than a syringe/pen treatment on a daily basis when the cost of the pump was amortised over a 4 year usage period was unlikely to be funded. In the UK, the NHS would not routinely fund insulin pumps.
78. Pumps are used by patients, including children and physically impaired patients, in their normal environment: they must withstand daily use and rough handling, including accidental drops. An effective and safe software-controlled insulin infusion pump design must minimise complexities and mitigate risks, in view of these human factors.
Disputed common general knowledge
80. As would be expected, there were disputes between the experts about the state of knowledge in the field, or specific factual or technical issues. Not all of them were to do with relevant CGK. I deal with disputes specific to the Patent or to obviousness where they arise. I deal with disputes about areas of CGK more generally below.
81. A number of the disputes raise the question as to whether certain aspects of mindset affect the CGK attributable to the skilled person. Mr Waugh QC helpfully drew my attention to the discussion of this issue by Floyd LJ in Koninklijke Philips N.V. v Asustek Computer Incorporation & Ors (where he considered both Hallen Co and another v Brabantia (UK) Ltd [1991] RPC 195) and Dyson Appliances Ltd v Hoover Ltd [2001] EWCA Civ 1440) [2019] EWCA Civ 2230) at [118]:
“These passages show that a commercially driven mindset can be a relevant aspect of the skilled person's common general knowledge. Thus, what the skilled person does in the light of a given prior disclosure has to be decided with that mindset in mind. If the technical differences from the prior art to the invention are trivial, then the mindset may not matter, but if more substantial changes are involved, the court may conclude that the reluctant and prejudiced skilled person would not make them. If the court reaches the conclusion that the claimed invention would be arrived at by the skilled person, there is no further hurdle to be crossed concerned with whether the invention would be perceived as likely to lead to sufficient commercial success to make its manufacture worthwhile.”
82. As emphasised by Floyd LJ and explained in those earlier cases, the mindset of the skilled person or team forms part of the CGK which would guide the team’s approach to any given problem and affect the steps it would be obvious for them to take. In the words of Aldous LJ in Dyson:
“The mantle of the skilled person is that of an actual skilled person. The purpose of assuming the mantle of the skilled person is to enable the decision as to what is obvious to be a decision based on actual facts. They include all the attitudes and perceptions of such a person.” [57]
83. With that in mind, I can deal with the competing views of the experts about the prevailing mindset within the industry at the priority date on the following disputed issues:
Mindset as to tubing
84. During cross-examination, Mr Causey discussed with Mr Tappin QC a number of difficulties with the use of tubed infusion sets and Mr Causey explained that in his view:
“There was no way to get rid of the tubing because there was no configuration of a pump and one imagined, or at least that we imagined where you could get rid of the tubing because that would have required the device to be body-adhered and we knew technology could not be body-adhered. It was simply too large. There was no way to imagine removing the tubing.”
85. When pressed by Mr Tappin QC, Mr Causey clarified that he was speaking from the perspective of MiniMed but, in assessing the relevance of his evidence as to the CGK, it must be recalled that MiniMed and Disetronic formed the bulk of the industry at the time and that for a number of years before the priority date all of those who were manufacturing and designing pumps had done so using a tubed configuration and with incremental improvements.
86. Mr Waugh QC and Mr Treneman also discussed the prevalence of tubing in the pumps available and widely known at the priority date. Their discussion was somewhat jumbled and this passage was one in which they were on occasion at cross purposes. However, after some discussions about the problems with the use of tubing and about the fact that it had continued to be used, Mr Treneman agreed that the use of tubing had been the accepted approach at the priority date.
87. In the light of the evidence given by the experts, I conclude that the CGK available to the skilled person at the priority date would have involved the use of external tubing in the design of a new insulin pump and this would have been seen as a good basis for further action in the field.
Mindset as to existing technology as a starting point
88. Mr Causey’s evidence was that “Most companies which had successful pumps on the market would begin their work on the next generation of pump by building on the existing technology that they already had.” He went on to elaborate that this was driven at least in part by patient confidence and trust in pumps that they knew and by safety considerations. Mr Treneman agreed that when beginning such a task the skilled person or team would have as part of their mental equipment the information that could be gleaned from existing products of a similar type. In the light of those agreements, I conclude that part of the CGK at the priority date would be the design choices, functionality and technology utilised in similar devices. For those with products already on the market, they might begin the design process from those specific products, but it does not follow that the skilled team would necessarily start with the specific attributes of a single existing product or family of products.
Mindset as to relevance of regulatory issues - a technical constraint on design or merely commercial consideration
89. It is clear that safety concerns were a technical constraint on design at the priority date and that those safety considerations would have formed a significant part of the “mental equipment” of the skilled team at the priority date. To the extent that regulatory concerns reflected safety issues, those would also have been relevant to the skilled team.
90. A more subtle point is the impact of regulatory procedures and processes on the mindset of the skilled team. Mr Causey gave evidence that the ease or otherwise of obtaining regulatory clearance would have driven the skilled team down a route which would involve limited departure from pre-existing products:
“So they were all tubed approaches and they were all very similar devices, with onboard displays. So there was such momentum for a pump that had an external infusion set, the cannula connected with a catheter tubing assembly and a large device with its user controls on the device, there was so much momentum that that was what the state of the art was at the time. So if a new intern (sic) wanted to then file a 510 K, he would have to then point to a predicate device such as our device and say it is substantially equivalent, which means very similar.”
91. In his written evidence he observed that:
“There would have been significant additional regulatory requirements for such a change. The sponsor company would need to convince regulators of the safety and efficacy of all of the new attributes and prove “substantial equivalence” of those new design features relative to currently approved predicates. The changes would require significant work and effort to persuade the regulators to approve the device as it was markedly different to the previous devices which had been approved. At the time, companies would submit regulatory approval requests based on incremental improvements.”
92. Mr Treneman’s evidence distinguished between safety considerations as such and regulatory considerations related to the ease of obtaining approval for new products.
93. It seems to me that regulatory constraints played both a technical and commercial role in the industry, forming part of the underlying matrix of considerations that drove a conservative approach to design. The fact that the regulatory considerations were in part motivated by commercial issues does not mean that they would not influence the approach of the skilled team or the steps that it would be obvious for the skilled team to take, as explained by Sedley LJ in Dyson:
“It is entirely in accordance with what we know about innovation that this commercial mindset will have played a part in setting the notional skilled addressee’s mental horizon, making a true inventor of the individual who was able to lift his eyes above the horizon and see a bag-free machine.” [89]
94. For that reason, I regard regulatory considerations as a part of the mindset of the skilled person in this field at the priority date meaning that existing technologies and products would, as mentioned above, be a good basis for future action.
A single mindset on design?
95. The parties disagreed whether the approach to design referred to above was shared by potential new entrants to the market. In this field, the skilled person would have been guided by principles of safety, would have been familiar with existing products and at a high level would start from existing technology with a safety first mindset. This would hold good whether the skilled person was employed by an existing company or by a new entrant. However, it would be unrealistic to find that the impact of these considerations would be the same in all circumstances. For example, the weight given to continuity would be less for the skilled person employed by a company without existing products or existing patients so it would be wrong to attribute that specific attribute to the notional skilled person. Equally, the specific weight given to particular design approaches would differ as between the employees of different companies, so again the weight to be given to specific design choices in the context of individual products would not be part of the mindset of the notional skilled person, although awareness of the choices previously made would be.
Catheters, cannulas and ports
96. The dispute was whether the tubing and/or the combination of tubing and infusion set (connected to a needle or cannula) was known as a catheter. This primarily goes to the construction of the prior art. Having reviewed the expert evidence, I do not consider that the skilled person would have had a settled view on the technical meaning of the word ‘catheter’ in the context of infusion pumps at the priority date.
97. Mr Treneman refers to the tubing as being a catheter, gave evidence that a catheter is ‘just a big tube’ and did not regard the cannula as forming part of the catheter. Mr Causey, on the other hand gave evidence that at the priority date a catheter would have been understood to have included not only the tube but also the needle or cannula. He also gave evidence that Disetronic had developed a catheter for use with a port and implanted surgically in a hospital, which indicated to me that the meaning of catheter was not so specific at the priority date as he suggested and that the meaning he advanced, while one possible meaning, would not necessarily have been regarded as a having a fixed meaning at the priority date. I do not regard it as being part of the CGK that the tubing and cannula set together would be referred to as a catheter, nor that cannula meant tubing plus cannula (i.e. a standard infusion set).
98. A further issue was whether it was known that catheters for insulin administration were used with implantable ports such as the Disetronic DiaPort. This primarily arose in Mr Causey’s oral evidence that the Disetronic DiaPort was introduced for surgical implantation in 1999. During cross-examination of Mr Treneman, Mr Waugh QC referred him to US 6071265 which described a ‘Catheter System for Skin passage Units’, was filed in March 1998 and reflected a foreign application in Switzerland with a priority date of 26 March 1997. The abstract described the invention as:
“Catheter system consisting of a sleeve, a catheter and a membrane partially arranged in the sleeve, which can be fixed in a skin passage unit in such a way that the catheter protrudes from the skin passage unit towards the interior of the body, wherein the individual components of the catheter system are inseparably connected to one another.”
99. Given the field in which the skilled person is deemed to have been active and interested, I consider it likely that this development in the delivery of insulin would have been of interest, and known, to the person skilled in the art at the priority date, albeit that it was in a field parallel to the specific task of designing an ambulatory insulin pump. As I discuss further below, the evidence of the experts is unclear as to whether this delivery method would have been regarded as a good basis for future action for the specific task at hand, given the surgical intervention required by the procedure. However, I find as a matter of fact on the evidence that it would have been known that catheters for insulin administration were used with implantable ports.
Problems with tubing
100. The dispute here was whether the inconvenience and problems associated with tubing (mentioned above) were perceived to be minor compared to the advantages of a CSII pump or whether tubing was regarded as one of the problems with CSII pumps. It is not clear that this is an issue of CGK rather than one of evidence as to the extent of a particular problem and its implications as discussed below in the context of the MiniMed prior art. In any event, both experts accepted that inconvenience and problems associated with tubing were known, even though solutions had been found to reduce some of those problems. The fact that the overall concept of ambulatory pumps and CSII was a significant advance does not mean that the tubing did not give rise to real problems, which would have been known to the skilled person.
Barcodes
101. Both experts agreed that barcodes were widely used at the priority date in some industries and Mr Treneman was of the view that “a system using a bar code and bar code reader would have been straightforward to implement”. The discussion about the use of barcodes arose largely in the context of obviousness rather than CGK and is considered further in that context below. As far as CGK is concerned, however, the evidence does not establish that barcodes were part of the CGK of the skilled person in this field at the priority date. The high point of the evidence on that point was during cross-examination of Mr Treneman when he gave evidence that he had used barcodes/QR codes on pharmaceutical products in 2000 and that they were becoming widely utilised at that time. As Mr Treneman no longer had samples of the way in which he had used barcodes, or of the way in which they had otherwise been used in the pharmaceutical sector at that time, it was not possible to establish the context for use, in particular whether they were used as a means of tracking and identifying products or batches or in the context of manufacturing and pairing devices and products.
102. In assessing the evidence on this particular issue I bear in mind the lengthy discussion of CGK in the very helpful judgment of Arnold J as he then was in KCI v Smith & Nephew. Arnold J cited the well-known passage from the judgment of Aldous LJ in Beloit Technologies Inc & Anor v Valmet Paper Machinery Inc & Anor [1997] EWCA Civ 993, [1997] RPC 489 at [494]-[495]. For context, I include that overview below, but the passage cited from the Judgment of Luxmoore J in British Acoustic Films Ltd v Nettlefold Productions Ltd [1936] 53 RPC 221 at [250] is of particular relevance as to how material which is common in one field may not be part of the CGK in another:
“The classic modern exposition of the law as to what constitutes common general knowledge is contained in the following repeatedly-cited passage from the judgment of Aldous LJ, building on earlier authorities, in Beloit Technologies Inc v Valmet Paper Machinery Inc [1997] RPC 489 at 494–495:
“It has never been easy to differentiate between common general knowledge and that which is known by some. It has become particularly difficult with the modern ability to circulate and retrieve information. Employees of some companies, with the use of libraries and patent departments, will become aware of information soon after it is published in a whole variety of documents; whereas others, without such advantages, may never do so until that information is accepted generally and put into practice. The notional skilled addressee is the ordinary man who may not have the advantages that some employees of large companies may have. The information in a patent specification is addressed to such a man and must contain sufficient details for him to understand and apply the invention. It will only lack an inventive step if it is obvious to such a man.
It follows that evidence that a fact is known or even well-known to a witness does not establish that that fact forms part of the common general knowledge. Neither does it follow that it will form part of the common general knowledge if it is recorded in a document. As stated by the Court of Appeal in General Tire & Rubber Co. v. Firestone Tyre & Rubber Co. Ltd. [1972] R.P.C. 457, at page 482, line 33:
‘The two classes of documents which call for consideration in relation to common general knowledge in the instant case were individual patent specifications and grave and ‘widely read publications’.
As to the former, it is clear that individual patent specifications and their contents do not normally form part of the relevant common general knowledge, though there may be specifications which are so well known amongst those versed in the art that upon evidence of that state of affairs they form part of such knowledge, and also there may occasionally be particular industries (such as that of colour photography) in which the evidence may show that all specifications form part of the relevant knowledge.
As regards scientific papers generally, it was said by Luxmoore, J. in British Acoustic Films (53 R.P.C. 221 at 250):
“In my judgment it is not sufficient to prove common general knowledge that a particular disclosure is made in an article, or series of articles, in a scientific journal, no matter how wide the circulation of that journal may be, in the absence of any evidence that the disclosure is accepted generally by those who are engaged in the art to which the disclosure relates. A piece of particular knowledge as disclosed in a scientific paper does not become common general knowledge merely because it is widely read, and still less because it is widely circulated. Such a piece of knowledge only becomes general knowledge when it is generally known and accepted without question by the bulk of those who are engaged in the particular art; in other words, when it becomes part of their common stock of knowledge relating to the art.”
And a little later, distinguishing between what has been written and what has been used, he said:
“It is certainly difficult to appreciate how the use of something which has in fact never been used in a particular art can ever be held to be common general knowledge in the art.”
103. I find that while the skilled team might have been aware of the use of barcodes in a general sense, the use of barcodes in insulin pumps has not been shown. The use of barcodes did not therefore form part of the relevant CGK at the priority date.
Wireless communication methods
104. The experts disagreed about whether methods for wireless communication between devices using Bluetooth and Zigbee were well-known, or whether such protocols would have been regarded by the skilled addressee as not yet commercially mature; and whether methods for wireless communication between devices were well-known, or whether developers would have had to design and develop their own wireless protocols.
106. Two other issues were raised as potentially forming part of the CGK:
· whether a mechanical actuation mechanism would typically be used for buttons with a mechanical pump drive system, while an electronic pump drive system would typically be used with electromechanical or electronic switches;
· whether the cost of electromechanical switches and circuit board traces would be insignificant or would add significant cost to a pump with an electronic motor system.
107. Insulet regarded both points as potentially forming part of the CGK. Roche disagreed. There is insufficient evidence to regard either issue as forming part of the CGK and they are better dealt with on the specific evidence as relevant.
The Patent
Overview
108. The Patent is entitled “Device and system for patient infusion” and explains:
“The present invention relates … to small, low cost, portable infusion devices that are useable to achieve precise, sophisticated, and programmable flow patterns for the delivery of therapeutic liquids to a mammalian patient.”
109. The Patent starts with a discussion of parenteral drug delivery. It describes existing ambulatory infusion pumps and their benefits, noting that they “allow control or programming via electromechanical buttons or switches located on the housing of the device”; that they “include visual feedback via text or graphic screens … and may include alert or warning lights and audio or vibration signals and alarms”; and that they “can be worn in a harness or pocket or strapped to the body of the patient”. Some of the drawbacks of currently available pumps are described including that such devices are “expensive, difficult to program and prepare for infusion, and tend to be bulky, heavy and very fragile” while their high cost has limited their adoption.
110. The Patent acknowledges prior art including PhiScience (WO 00/29047 published 25 May 2000), stating that it “discloses a device and a method for supplying medicaments by a mobile, portable, non-implantable means with wireless operation or programming”, and MiniMed (WO 00/10628 published 2 March 2000), which it says “discloses an infusion system with remote programming, bolus estimator and vibration alarm capabilities and containing a fluid that is expelled through an outlet in a reservoir and housing, and then into a body of a user through tubing and a set”. The Patent concludes its description of the prior art as follows: “None of the above disclose a programmable and adjustable infusion system that is precise and reliable and can offer clinicians and patients a small, low cost, light weight, simple to use alternative for parenteral delivery of liquid medicines”.
Summary of the Invention
111. The invention is summarised by reference to its key objectives and embodiments. The patentee describes the need for a “a sophisticated ambulatory infusion device that can be programmed to reliably deliver variable flow profiles of liquid medications, yet is small, light weight and low cost … Smaller and lighter devices are easier to carry and are more comfortable for the patient, even allowing the device to be adhesively attached to the patient’s skin similar to a transdermal patch”. The benefits of such a device are said to include “cost reductions significant enough to make the entire device disposable in nature, being replaced as frequently as every two to five days”. The Patent explains why disposability is seen as being advantageous.
112. Having set out the objectives, the Patent explains how those are to be achieved, summarising the main elements of the device:
“Embodiments of the present invention, therefore, provide a device for delivering fluid to a patient, including an exit port assembly adapted to connect to a transcutaneous patient access tool, a dispenser for causing fluid from a reservoir to flow to the exit port assembly, a local processor connected to the dispenser and programmed to cause a flow of fluid to the exit port assembly based on flow instructions from a separate, remote control device, and a wireless receiver connected to the local processor for receiving the flow instructions from a separate, remote control device and delivering the flow instructions to the local processor. The device also includes a housing containing the exit port assembly, the dispenser, the local processor, and the wireless receiver. The housing is free of user input components for providing flow instructions to the local processor in order to reduce the size, complexity and costs of the device, such that the device lends itself to being disposable in nature.”
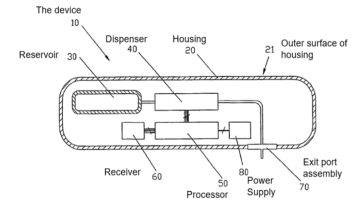
114. The Patent explains that the invention will allow flow instructions for a basal rate and bolus volume to be programmed and that in some embodiments “the device includes [at] least one user interface component accessible from an exterior of the housing for causing a predetermined volume of fluid to flow for a predetermined period, independently of the local processor” and may also include a user interface component “for occluding flow to the exit port assembly”.
115. The remote control device is connected wirelessly to the local processor of the pump device via a two-way transmitter. This also enables the housing to be “free of user output components for providing information from the local processor”, since such information can be displayed on the remote control instead.
116. The Patent explains that “these aspects of the invention together with additional features and advantages thereof may best be understood by reference to the following detailed descriptions and examples taken in connection with the accompanying illustrated drawings”. Paragraphs [0023]-[0050] provide a brief description of the drawings and the detailed description of the invention follows. Each of the figures is described in detail but I briefly summarise the key attributes of those which are relevant below.
117. Figure 1 shows a first exemplary embodiment. Figure 2 shows a remote control device for use with the fluid delivery device.
118. Figures 5 and 5a show an embodiment which includes a second reservoir and second dispenser; together with “a transcutaneous patient access tool comprising transcutaneous micropenetrators 75 connected to the exit port assembly” and an adhesive layer for securing the device to a patient’s skin.
119. Figures 6 and 6a show an embodiment with two adhesive layers for attaching and reattaching the device to a patient’s skin and it is explained that “Contrary to the claimed invention, a needle connection tubing 73 terminating in a skin penetrating cannula (72) is shown connected to the exit port assembly”.
120. Figures 8 and 8a show an embodiment including a modular sub-assembly for the electronic components and the reservoir which are in separate sub-assemblies inserted within the fluid delivery device and the provision of a barcode on the device which can be read by a barcode scanner on the remote control.
121. Figure 9 shows an embodiment of a fluid delivery device and Figure 9a shows an infusion set compatible with that device. It is explained that “Contrary to the claimed invention, Fig. 9a shows a standard transcutaneous infusion set 400 consisting of a penetrating cannula 405, usually consisting of a needle bent to ninety degrees, a flexible tubing 404 and a Luer connector 401, which includes standard threads 402.”
122. Figures 10 and 10a show further embodiments including means for stopping flow without use of the remote control device.
123. Figures 11 and 11a show further embodiments including a means for delivering a fixed amount of fluid (a bolus) without use of the remote control.
124. Figures 12 and 12a show an embodiment of a remote control device including a visual display on the remote control.
125. Figure 13 shows an embodiment of a fluid delivery device and Figures 13a-13c show, respectively, a remote controller, an insulin cartridge and an infusion set to be combined with that device as part of a kit.
126. In addition to the paragraphs describing and expanding on the figures, the Patent then contains, just before the claims, a series of paragraphs which appear to be general, rather than related to any particular embodiment.
Claims in Issue
127. Insulet relies on five claims as independently valid and infringed. The trial proceeded on the basis of the claims as proposed to be unconditionally amended. The parties agreed to use a renumbered format, which is adopted throughout this judgment. A document setting out the Claims in Issue broken into integers (as agreed by the parties and described at paragraph 25 of the PTR Order) is attached as Appendix B. Claim 1 is set out below, with the integer numbering added for convenience. It is followed by a description of the other claims.
Claim 1
128. [1A] A device (10) for delivering fluid to a Type I diabetic patient, comprising:
· [1B] an exit port assembly (70) adapted to connect to a transcutaneous patient access tool;
· [1C] a dispenser for (40) causing fluid from a reservoir (30) to flow to the exit port assembly;
· [1D] a local processor (50) connected to the dispenser and programmed to cause a flow of fluid to the exit port assembly based on flow instructions;
· [1E] a wireless receiver (60) connected to the local processor for receiving flow instructions from a separate, remote control device and delivering the flow instructions to the local processor; and
· [1F] a housing (20) containing the exit port assembly, the dispenser, the local processor, and the wireless receiver;
[1G] wherein the housing is free of user input components for providing flow instructions to the local processor; and
[1H] wherein the transcutaneous patient access tool is integrated into the exit port assembly; and
[1I] wherein the reservoir (30) is contained in the housing (20) and has a volume in the range of 2 to 3 ml.
Amended Claim 2 (original Claim 37)
129. This claim is relied upon as shown in amended Claim 2 in Appendix A. It is dependent on original Claims 1 and 38 (original Claim 37), such that there is no constraint on reservoir volume (feature I of Claim 1). Claim 2 claims a system with a “remote control device” which is separate to the “fluid delivery device”. The claimed remote controller has a processor, user interface components allowing the user to provide flow instructions to the processor, and a transmitter for transmitting those instructions to the fluid delivery device. It adds the requirement of a proximity alarm.
Amended Claim 3 (original Claims 40 and 41)
130. This claim is relied upon in the written-out form which is shown in Appendix A. It corresponds to original Claim 40, and claims a kit comprising a single remote control device and a plurality of fluid delivery devices. This claim provides two additional aspects over Claims 1 and 2:
· It provides for the supply of multiple fluid delivery devices to the user as part of an overall package with a single remote control device;
· It includes the additional feature of the fluid delivery devices having a barcode which can be paired to the remote control using a barcode scanner on the remote control.
Amended Claim 43
131. This claim (originally Claim 42), depends on amended Claims 1, 2 or 3. The claim is to a device, system or kit in which the housing of the fluid delivery device is free of user output components for providing flow information from the local processor to a user and in which the fluid delivery device receives at least some flow instructions from the separate remote control device.
Amended Claim 45
132. This claim (originally Claim 44 as granted) is to a system comprising (a) a fluid delivery device according to Claim 1 for attachment to a skin surface of a patient; and (b) a remote control device separate to the fluid delivery device with user input and output components. There is a wireless transmitter and receiver in both the remote control and the fluid delivery device, thereby enabling two-way communication between them of flow instructions and flow information.
Legal principles - construction
133. The general principles of claim construction are well-established, although this did not prevent dispute as to some particular issues of claim interpretation. Where those disputes are relevant to my conclusions, I deal with them specifically below.
134. The starting point (to which I was referred by counsel for both parties) is the summary by Jacob LJ in Virgin Atlantic Airways Ltd v Premium Aircraft Interiors Group [2009] EWCA Civ 1062, per Jacob LJ at [5]:
“The task for the court is to determine what the person skilled in the art would have understood the patentee to have been using the language of the claim to mean. The principles were summarised by Jacob LJ in Mayne Pharma v Pharmacia Italia [2005] EWCA Civ 137 and refined by Pumfrey J in Halliburton v Smith International [2005] EWHC 1623 (Pat) following their general approval by the House of Lords in Kirin-Amgen v Hoechst Marion Roussel [2005] RPC 9. An abbreviated version of them is as follows:
(i) The first overarching principle is that contained in Article 69 of the European Patent Convention;
(ii) Article 69 says that the extent of protection is determined by the claims. It goes on to say that the description and drawings shall be used to interpret the claims. In short the claims are to be construed in context.
(iii) It follows that the claims are to be construed purposively—the inventor’s purpose being ascertained from the description and drawings.
(iv) It further follows that the claims must not be construed as if they stood alone—the drawings and description only being used to resolve any ambiguity. Purpose is vital to the construction of claims.
(v) When ascertaining the inventor’s purpose, it must be remembered that he may have several purposes depending on the level of generality of his invention. Typically, for instance, an inventor may have one, generally more than one, specific embodiment as well as a generalised concept. But there is no presumption that the patentee necessarily intended the widest possible meaning consistent with his purpose be given to the words that he used: purpose and meaning are different.
(vi) Thus purpose is not the be-all and end-all. One is still at the end of the day concerned with the meaning of the language used. Hence the other extreme of the Protocol—a mere guideline—is also ruled out by Article 69 itself. It is the terms of the claims which delineate the patentee’s territory.
(vii) It follows that if the patentee has included what is obviously a deliberate limitation in his claims, it must have a meaning. One cannot disregard obviously intentional elements.
(viii) It also follows that where a patentee has used a word or phrase which, acontextually, might have a particular meaning (narrow or wide) it does not necessarily have that meaning in context.
(ix) It further follows that there is no general “doctrine of equivalents.”
(x) On the other hand purposive construction can lead to the conclusion that a technically trivial or minor difference between an element of a claim and the corresponding element of the alleged infringement nonetheless falls within the meaning of the element when read purposively. This is not because there is a doctrine of equivalents: it is because that is the fair way to read the claim in context.
(xi) Finally purposive construction leads one to eschew the kind of meticulous verbal analysis which lawyers are too often tempted by their training to indulge.”
135. Counsel for Roche pointed out, and I agree, that (ix) and (x) must be read subject to the Judgment of the Supreme Court in Actavis UK Limited and others v Eli Lilly and Company [2017] UKSC 48, but the overall approach set out by Jacob LJ remains good law and, as Lord Neuberger subsequently explained in Actavis v Eli Lilly at [10], the overall exercise is approached in a way similar to construing a contract:
“As Floyd LJ explained in the Court of Appeal, the appeal raises the issue of the correct approach under UK law (and the law of the three other states) to the interpretation of patent claims, and in particular the requirement of EPC 2000 to take account of "equivalents", and also the extent to which it is permissible to make use of the prosecution history of a patent when determining its scope. The issue on the cross-appeal is rather more fact-specific, namely whether the application of the law of contributory infringement justifies a finding of indirect infringement in this case.”
136. Given the explicit reference to the principles of contractual construction by Lord Neuberger, it is worth repeating for the sake of convenience what was said by Lord Hodge in the leading case on that question Wood v Capita Insurance Services Ltd [2017] [2017] UKSC 24 at [8]-[15]:
“The court’s task is to ascertain the objective meaning of the language which the parties have chosen to express their agreement. It has long been accepted that this is not a literalist exercise focused solely on a parsing of the wording of the particular clause but that the court must consider the contract as a whole and, depending on the nature, formality and quality of drafting of the contract, give more or less weight to elements of the wider context in reaching its view as to that objective meaning. In Prenn v Simmonds [1971] 1 WLR 1381 (1383H-1385D) and in Reardon Smith Line Ltd v Yngvar Hansen-Tangen [1976] 1 WLR 989 (997) , Lord Wilberforce affirmed the potential relevance to the task of interpreting the parties’ contract of the factual background known to the parties at or before the date of the contract, excluding evidence of the prior negotiations. When in his celebrated judgment in Investors Compensation Scheme Ltd v West Bromwich Building Society [1998] 1 WLR 896 Lord Hoffmann (pp 912-913) reformulated the principles of contractual interpretation, some saw his second principle, which allowed consideration of the whole relevant factual background available to the parties at the time of the contract, as signalling a break with the past. But Lord Bingham in an extra-judicial writing, A new thing under the sun? The interpretation of contracts and the ICS decision Edin LR Vol 12, 374-390, persuasively demonstrated that the idea of the court putting itself in the shoes of the contracting parties had a long pedigree.” [10]
137. Since the Supreme Court judgment in Actavis v Eli Lilly, it has been confirmed that the ‘normal’ approach to construction of a patent is that of ‘purposive construction’. The correct approach was explained by Arnold J (as he then was) in Generics (UK) Ltd v Yeda Research and Development Co Ltd [2017] EWHC 2629 (Pat) at [138]:
“… As has often been pointed out, patents differ from commercial contracts in two key ways. First, a contract is (at least in principle) a bilateral statement agreed between the contracting parties, whereas a patent is a unilateral statement made by the patentee and addressed to the class of persons represented by the person skilled in the art. Secondly, whereas a contract is a document containing promises by the contracting parties to each other (in some cases for the benefit also of third parties), a patent is a document which describes and claims an invention for the purposes of establishing a legal monopoly with regard to that invention. One cannot rationally interpret a patent claim without taking these matters into account. Moreover, I do not consider that Lord Neuberger can have meant anything different, even though he appears to have eschewed the expression “purposive construction” when describing the correct approach. On the contrary, in the passages relied upon by counsel for the Claimants, he expressly stated that a patent was to be interpreted through the eyes of the person skilled in the art and that the exercise involved interpreting the words of the claim in context. The context must include the very purpose for which the document exists, namely to describe and claim an invention.” [138]
and subsequently approved in, for example, Icescape Ltd v Ice-World International BV [2018] EWCA Civ 2219 at [60].
Claim interpretation
138. The Claims in Issue are described above. A number of questions of claim interpretation arise, some of which are common to all claims and some of which are specific to particular claims. In approaching interpretation, I have in mind:
· the patentee’s purpose as disclosed by the claims;
· interpreted with the assistance of the description and drawings;
· without undue literalism; but also remembering that
· the language used in the claims ultimately delineates the territory claimed by the patentee; and that
· the Patent is addressed to a person skilled in the relevant art.
139. In this task I have been assisted by the experts, but ultimately, other than in respect of words with a technical meaning which is appropriate in the context of the Patent, the question of interpretation is for the Court.
140. The claims are to be interpreted bearing in mind the overall purpose of the claimed “device for delivering fluid to a Type I diabetic patient” with the assistance of the description and drawings. Broadly speaking, the description explains that the patentee intends to provide “small, low cost, portable infusion devices that are useable to achieve precise, sophisticated, and programmable flow patterns for the delivery of therapeutic liquids to a mammalian patient”. The concepts of small size, low weight and low cost are a constant theme throughout the patent description and are reflected in the drawings. The importance of these attributes is explained in the description and sheds further light on the intention of the patentee and the purpose of the claims.
141. The Patent first describes the need for a “a sophisticated ambulatory infusion device that can be programmed to reliably deliver variable flow profiles of liquid medications, yet is small, light weight and low cost … Smaller and lighter devices are easier to carry and are more comfortable for the patient, even allowing the device to be adhesively attached to the patient’s skin similar to a transdermal patch” and subsequently the description explains the patentee’s concept of the invention more fully: “Embodiments of the present invention, therefore, provide a device for delivering fluid to a patient, including an exit port assembly adapted to connect to a transcutaneous patient access tool, a dispenser for causing fluid from a reservoir to flow to the exit port assembly, a local processor connected to the dispenser and programmed to cause a flow of fluid to the exit port assembly based on flow instructions from a separate, remote control device, and a wireless receiver connected to the local processor for receiving the flow instructions from a separate, remote control device and delivering the flow instructions to the local processor. The device also includes a housing containing the exit port assembly, the dispenser, the local processor, and the wireless receiver. The housing is free of user input components for providing flow instructions to the local processor in order to reduce the size, complexity and costs of the device, such that the device lends itself to being disposable in nature.”
142. I find the patentee’s overall purpose to be to stake a claim over devices which are controlled by a remote controller, conceptually small, lightweight, low cost, simple to use, reliable, programmable, comfortable, capable of being adhesively attached to the patient’s skin and such that the device lends itself to being disposable in nature.
143. Against that background, the integers in respect of which there is a dispute as to construction between the parties are dealt with in turn below.
“an exit port assembly (70) adapted to connect to a transcutaneous patient access tool” (all claims in issue - Integers 1B, 2C, 3C, 42A, 45B)
144. The parties’ submissions dealt with aspects of this integer at different points, as some of the words used arise in different integers and contexts. Most submissions arose in the context of the infringement arguments and will be dealt with below. Two aspects of the integer are important to the construction of the Patent as a whole: exit port assembly (“EPA”) and transcutaneous patient access tool (“TPAT”).
EPA
146. Mr Causey explained that in his view the EPA: “…connects the flow paths within the pump to the flow paths outside the pump and into the user of the device” and this was not disputed.
147. The principal dispute was whether Mr Causey was correct in suggesting that EPA should be understood in the light of the depiction of EPAs in the figures showing the exemplary embodiments (or by the fact that in the claim integer itself the reference number 70 is used - which also appears in those figures). This was submitted by Mr Tappin QC to be an illegitimate use of the numerals in the claim to construe the claim as having a limitation. Mr Tappin QC relied primarily on Virgin Atlantic Airways v Premium Aircraft Interiors at [17] and [60] to make good this point, but ultimately I did not regard the figures to be of particular assistance to construction in any event.
TPAT
“a housing (20) containing the exit port assembly, the dispenser, the local processor, and the wireless receiver” (all claims in issue - Integers 1F, 2G, 3G, 42A, 45B)
152. In summary, Insulet’s position is that the housing is formed by the external shell of the device, when assembled for use, which encloses its internal components. Mr Causey’s evidence was:
“I have been asked to identify the “housing” in the Solo device. I consider the Solo has a housing when the components are joined and ready for its intended use (to deliver insulin to the user). The Solo device, when ready to be used, is an integrated assembly of the micropump (which itself is formed by the attachment of the pump base and filled reservoir) and the pump holder, at which stage the cannula is integrated into the exit port of the micropump. These parts are clearly designed to fit together and operate as a single integrated unit. The housing is the integrated external shell of the assembled device which encloses the internal components of the device.”
and that there is nothing in the claim language that requires a more limited construction as long as the housing serves the purpose of containing the claimed subcomponents within its boundaries.
153. Roche’s position is that this integer requires a single housing which contains each of the specified components and does not encompass a situation in which there are two or more ‘separate housings’. Mr Treneman accepted in cross-examination that the essential quality of a housing is that when assembled it protects the components within, and that the housing claimed by the Patent should is required to have only a generally smooth outer appearance:
“Q. The essential quality of a housing is that when assembled it protects the internal components; correct?
A. Yes.
Q. It should have a generally smooth outer appearance that will not snag jewellery but can be opened as needed to refill the reservoir or change the battery, for example; correct?
A. Yes.”
154. Roche nevertheless submitted that, in context, where the purpose of the Patent is to produce a cheap, simple, disposable device which can remain in place on the patient’s body for a few days (requiring it to withstand events such as showering) and then be discarded, the description envisages a single device which is intended to be disposable in nature. Roche relied particularly on paragraph [0059] of the Patent:
“The lack of user interfaces also allows the housing outer surface 21 of the device 10 be relatively smooth, thereby simplifying cleaning and preventing jewelry or clothing items such as sweaters from catching on the device”.
155. Roche submitted that this would not be an apt description of a multi-part design, which would not be seamlessly joined together and would be harder to clean and that therefore the word housing must interpreted as requiring a singular contiguous shell at the time when the product is delivered to the user. Roche relied on the repeated references throughout the description to “the” (or “a”) housing, singular.
156. In support of its proposed construction, Roche also drew my attention to a decision of the Dusseldorf Court considering the meaning of ‘housing’ in the context of an infringement action against a different defendant (Insulet v Medtrum, Landgericht Düsseldorf judgment 4c O 33/19 dated 13 August 2020).
157. Considering the submissions:
· As to the way in which the requirement that the device be cheap, simple and wearable for a few days before being discarded must inform the construction of the word housing, the key issue is whether the evidence suggests on balance that the patentee required a single outer shell as of the date of manufacture and whether the skilled person would have read those requirements as mandating a single housing at the time when the device was delivered to the user. Having considered the expert evidence in the round, it is not clear that by requiring that the device have these attributes the patentee intended that the outer casing must be a single part when supplied to the user. Such attributes might be considered by the skilled user to be achievable using two pieces or more. Looking at the language on a purposive basis and in the context of the evidence as a whole, the key passage on which Roche relies places greater emphasis on the removal of user interfaces from the housing as achieving the desired outcomes than on the design of the housing itself. The requirement that the surface of the device must be ‘relatively smooth’ and therefore easier to clean, seems to be foreseen as following from the reduction in user interfaces on the surface of the housing rather than from the fact that the housing might initially formed from only one piece rather than two or more pieces.
· Roche’s reliance on the textual reading of ‘a housing’ as necessarily meaning that the housing must be formed of a single part did not persuade me that it really went to the intention of the patentee. In my view it sought to stretch the language of the claim to read into it an unintended limitation rather than following it and is syntactic analysis of the type which must now be avoided.
· The high point of Roche’s arguments is that the Patent clearly intends that the device have a high degree of disposability and that the nature of the housing is relevant to this consideration. However, having further reviewed the portions of the specification to which Roche referred, I conclude that the principal issue to which those paragraphs are directed (in the context of disposability) is the low cost nature of the device and the removal from it of expensive components such as the majority of the user interface, so that it would be cost effective and economically rational to dispose of it regularly, rather than whether the outer housing was or was not formed of one piece.
158. Looking at the specific claim language through the prism of the Patent as a whole, and trying to divine what meaning would have been apparent to the skilled person, I conclude that the skilled person would understand the Patent to claim a device with a covering which would serve the purposes of:
· containing the components safely and securely in the normal course of a user’s daily life, having regard to the intention that it be wearable and suitable for direct attachment to the patient’s skin;
· maintaining a relatively smooth, rather than jagged or pitted, surface on which user interfaces had been reduced or eliminated, thereby assisting with cleaning; and
· enabling the device to meet its purposes of being low cost and light weight, suitable for wearing and potentially disposable.
“wherein the housing is free of user input components for providing flow instructions to the local processor” (all claims in issue - Integers 1G, 2H, 3H, 42A, 45B)
160. The dispute concerns whether this integer requires that the housing not contain any user input components whatsoever, or whether it can have some such components. Both experts expressed helpful views, which I refer to as relevant below. Neither contended that any aspect of the integer was a term of art.
161. In summary, Roche submitted that there can be no real dispute about the interpretation of this integer: the housing must be free of - meaning ‘have no’ - user input components which can provide flow instructions to the local processor of the fluid delivery device.
162. Roche acknowledges that the Patent envisages that the housing may have some user input components (such as the stop and bolus buttons shown in Figures 10, 10a, 11 and 11a and described at paragraphs [0018] and [0088]-[0094]) and also that the presence of such a stop button is specifically envisaged by Claim 9. However, Roche draws a distinction between those buttons, which it says provide flow instructions other than to the local processor and which are mechanical, and user input components which do provide flow instructions to the local processor and which are electro-mechanical. Roche submits that, on any interpretation of the claim, it is clear that the removal of user interface components for providing flow instructions to the local processor is integral to the patentee’s purposes and that the description makes clear that this means electro‑mechanical buttons.
163. Insulet notes that the parties agree that Integer 1G does not require the housing to be free of all user input components whatsoever. Insulet submits that the chief difference lies in the type of user input component which is permissible and in the degree of freedom to include them permitted by the claim. Insulet says that on a purposive construction:
(i) the term ‘free of’ is not an absolute term when read purposively against the objects of the invention; and
(ii) Integer 1G is not a limitation based on the type of component or button.
164. Before reviewing the chief arguments of the parties on this issue, it is worth being clear about the meaning of some of the specific words and phrases used in the integer. Mr Causey’s evidence was that:
“EP 764 refers to “user input components”, “user output components” and “user interfaces”. These are not specific terms of the art but the skilled person would understand in the context of EP 764 that:
(A) user input components include means by which instructions are provided by the user to the system, such as control pads, buttons, switches. See for example [0056].
(B) user output components include means by which information is provided from the system back to the user such as display screens. See for example [0057].
(C) user interfaces include both user input components and user output components. See for example [0059].
It also seems to me that the “user” referred to in EP 764 is the user of the pump device; that is, the patient who is treating and managing their diabetes (and not a healthcare professional or remote monitoring and control station, as referred to in some of the prior art documents).
EP 764 also refers throughout to flow instructions and flow information. Neither of these are specific terms of the art but they describe two broad categories of information in pump systems. In the context of EP 764:
(A) “flow instructions” are instructions provided via user input components, which are delivered to the local processor of the fluid delivery device and determine the flow of fluid executed by the device. In the EP 764 system, these user input components are present on a separate remote control device. See for example [0015] and [0052]. The flow instructions can include instructions for both the rate of fluid flow (basal rate) and a predetermined volume of fluid flow (bolus volume [see 0016 and 0017].
(B) “flow information” is information provided by the local processor concerning the fluid flow which has taken place within the fluid delivery device and includes both basal and bolus insulin flow. In the EP 764 system this information can be transmitted to the remote control and can be displayed using user output components. See [0021].”
165. Mr Treneman agreed with much of what Mr Causey had said but disagreed on one or two points:
“As regards paragraph 106 (B), I agree that a display screen would be one example of a user output component. However, information could also be provided from the system back to the user in the form of sound or haptic signals, as was already done in insulin pumps in the 1990s. The components providing such sound or haptic signals would also be user output components.”
166. To the extent that the differences are relevant I will deal with them below.
167. Against that background, Insulet argued, in summary, that Roche’s position was untenable as it was based on an unacceptably literal approach to Integer 1G, and that on a purposive approach it was apparent that the skilled person would understand both that the integer should be interpreted to allow some user input components such as stop buttons and bolus buttons, and that there was no basis to distinguish between various types of user interface component as Roche sought to do. Insulet advanced five main arguments in support of its proposed construction.
The patentee’s purpose
168. Insulet referred first to the explanation at paragraph [0059] as disclosing the patentee’s purpose in relation to Integer 1G. When discussing the skilled person’s understanding of Integer 1G and the help that could be gained from that paragraph Mr Causey states:
“The important requirement is that the fluid delivery device should be controlled remotely with a separate and re-usable remote control device which contains the expensive electronics and removes significant weight and volume from the fluid delivery device itself.”
169. Insulet submitted that the patentee’s purpose was therefore to enable substantial reductions in cost, size and weight, to ensure a relatively smooth housing outer surface, to simplify design and to make the device more flexible and resistant to damage, such that the device lends itself to being small, wearable and disposable in nature. Read against that purpose, and given that the Patent specifically envisaged the presence of some buttons on the outer hosing of the device, Insulet contended that a reading which distinguished between mechanical and electromechanical buttons would be too literal and that the skilled person would have concluded that some buttons would be acceptable as long as they did not undermine the overall purpose of the patentee.
170. Roche argued that the purpose of the integer within the claim must be considered, submitting that care in identifying the inventor’s purpose is particularly critical when construing an integer such as 1G, and pointing out that the description in paragraph [0059], which goes directly to the purpose of this part of the claim is very specific about the removal of user interfaces, consistent with the claim language. Paragraph [0059] is set out in full below:
“The lack of user interfaces, such as electromechanical switches on the fluid delivery device 10, results in substantial reductions in the cost, the size, and the weight of the device 10. The lack of user interfaces also allows the housing outer surface 21 of the device 10 to be relatively smooth, thereby simplifying cleaning and preventing jewelry or clothing items such as sweaters from catching on the device. Since the remote control device 100 also includes a visual display 110, the fluid delivery device 10 can be void of an information screen, further reducing cost, size and weight. Lack of user interfaces, such as electromechanical switches and information screens, greatly simplifies the design of the fluid delivery device 10 and allows the device 10 to be made more flexible and resistant to damage.”
171. Roche argues that, far from being a general explanation of an overall purpose of reducing weight, complexity, cost and size, this paragraph explains the patentee’s expectation that the purpose would be achieved through removing user interfaces, whether user input components such as electromechanical switches or other user interfaces such as information screens. The inventor’s purpose, Roche submits, is not merely to achieve the benefits which are the overall purpose of the Patent, but also to explain that this will be achieved by the means which are specifically identified in Integer 1G: i.e. removing user interfaces so that there is a ‘lack of’ such components on the device, or so that the ‘housing is free of’ those components. Roche argues that the language of the claim is wholly consistent with the wording in the specification and that it would be wrong to ignore the clear and deliberate limitation which is contained both in the claim and in the specification.
172. Mr Treneman’s evidence was that paragraph [0059] identifies the absence of user input components as being a key means by which a small, lightweight, low cost, disposable pump can be achieved. Mr Treneman also noted several other passages in the description which identify the lack of user input components as key: paragraphs [0015], [0052] and [0056]. Mr Treneman concluded that his view of how the skilled person would read the Patent was that “… the teaching of the Patent is that it is the removal of all the electromechanical switches from the device that allows the electronics to be moved from the fluid delivery device to the remote control, and significant weight and volume to be removed from the fluid delivery device itself.” When this conclusion was put to Mr Causey in cross-examination by Mr Tappin QC, he agreed that, as stated, Mr Treneman’s conclusion was correct.
Absolute limitation?
173. Insulet argues that Integer 1G should be interpreted not as an absolute exclusion of any means of user input, but as a relative limitation which sufficiently removes the user input components to avoid the disadvantages of the prior art such as cost, bulk, weight, expense, risk of buttons catching on clothes and so on). Insulet relies on the description at paragraph [0114]:
“[0114] Although exemplary embodiments of the invention have been shown and described, many changes, modifications and substitutions may be made by those having ordinary skill in the art without necessarily departing from the scope of this invention. For example, the preferred fluid delivery device is intended to be low cost, light weight, simple to use and potentially disposable by removing a majority of the user interface, including electromechanical switches, from the fluid delivery device, and including a separate controller to replace those functions. A reservoir, fluid dispenser, transcutaneous fluid administration means, solid state electronics and wireless communications are included in the fluid delivery device to perform its intended function. While various means for reservoir construction, pressurization means, fluid pumping means, fluid metering means, transcutaneous delivery, electronic control and wireless communications have been discussed in this application, alternatives to each of these areas can be made without departing from the scope of the invention as defined in the claims.”
174. Insulet submitted that this description is consistent with Integer 1G not imposing an absolute restraint and relies on a series of cases which it submits hold that absolute words may take on a less absolute meaning. The cases relied on included Nikken Kosakusho Works v Pioneer Trading Co [2005] FSR 15; Minnesota Mining & Manufacturing Co v Plastus Kreativ AB [1997] RPC 737, 752; Unilin Beheer BV v Berry Floor NV (Court of Appeal) [2004] EWCA Civ 1021; and others in a similar vein.
175. Unsurprisingly, Roche disagreed.
176. Roche’s position on paragraph [0114] was that Insulet’s reading would be inconsistent with the statements earlier in the description about removing all user input components from the housing of the device, and the reasons for doing so (as discussed above). Roche submitted that the passage relied on by Insulet is saying simply that a majority of the user interface (as a whole, i.e. both input and output components) should be removed from the device, and that at least electromechanical switches should be removed to the remote controller. That would be entirely consistent with the earlier statements in the description about removing such elements from the device, as well as the option of retaining mechanical buttons. Roche also argued that this paragraph is saying simply that changes can be made to the exemplary embodiments for aspects such as reservoir construction, without departing from the invention as defined in the claims.
177. Roche argued that the authorities cited by Insulet in support of the proposition that “a word with absolute overtones can be made to bear relative ones” are not apposite and that it is necessary to contrast:
(i) a situation where the claim language relates to uncountable, continuous parameters, and the claim uses words that are capable of involving a question of degree; with
(ii) a situation where claim language relates to an integral, countable and small number of components, and the claim uses words that in context are incapable of involving a question of degree.
178. Roche’s position was that authorities relied upon by Insulet fall into the former category and the present case into the latter, there being no suggestion in the claim language that any user interface components for providing flow information to the local processor could remain on the outer housing.
179. Roche further submitted that to construe the language of Integer 1G in context required a review of what was known at the time, as disclosed in the description, and therefore what the claims would not teach. The pre-priority infusion pumps which formed part of the CGK included a small number of electromechanical buttons (three in the case of the Disetronic H-TRONplus, four in the case of the MiniMed 508). Roche pointed out that paragraph [0006] refers to existing devices that “allow control and programming via electromechanical buttons or switches located on the housing of the device” while paragraph [0015] distinguishes the device claimed by the invention by saying that the “housing is free of user input components for providing flow instructions to the local processor in order to reduce the size, complexity and costs of the device”. This is a point re-emphasised in paragraphs [0052], [0056] and [0059] (already referred to above).
Safety issues and buttons generally
181. Insulet’s third set of submissions went to safety aspects of the device and rested on the fact that the Patent permits a housing which is otherwise free of user interfaces to incorporate a bolus or emergency stop button as a safety mechanism. This is described in paragraph [0018], further explored in paragraphs [0088]-[0089] and specifically envisaged in Claim 9. It was submitted that safety is an important aspect of design and that the skilled person would consider it risky to remove safety bolus or stop controls. Insulet went on to submit that, as such buttons were envisaged, it would be irrelevant whether they were electro-mechanical or mechanical.
182. Roche disagreed on the basis that:
(i) Insulet fails to make the distinction between mechanical buttons and electromechanical user input components for providing flow instructions to the local processor; and
(ii) simply because the Patent envisages the housing having buttons which do not provide flow instructions to the local processor does not mean that the claims allow the presence of buttons which do. Roche submitted that, whenever buttons are described and shown on the housing at paragraphs [0088]-[0094] and in Figures 10 and 11, they are mechanical buttons, serving to re-emphasise the distinction that the Patent is making between what is and is not claimed.
183. Roche submits that, in the light of the above, it is wrong to suggest that the Patent does not exclude bolus buttons that engage the local processor. Roche emphasises that the Patent excludes such controls, including by way of a specific limitation in the claim, and accordingly when it teaches any emergency buttons, they are taught as mechanical - as for example: “the user can press a mechanical bolus button” (paragraph [0089]).
Buttons in dependent claims
184. Insulet’s fourth line of argument refers to Claims 9 and 23 (as proposed to be amended) - which claim the mechanical stop and bolus buttons, respectively, that are depicted in Figures 10 and 11 - and also to Claim 43 (as proposed to be amended). It argues first that, as these are limitations on Claim 1, Claim 1 must be construed widely enough to encompass them; and, in the case of Claim 43, that as it envisages “… at least some of the flow instructions from a separate, remote control device”, this is consistent with at least some flow instructions coming from components on the device itself.
185. Roche’s replies were: first, as Claims 9, 23 and 43 are dependent claims, their role is to narrow the independent claim (as Claims 9 and 23 do) by introducing an additional requirement of a mechanical button if those features are adopted. Claim 43 is said to be consistent with the overall teaching of the Patent as it envisages that some flow instructions might be delivered by mechanical buttons rather than the remote control.
Patentee’s incentive for limitation
186. Insulet’s final point on Integer 1G is a further iteration of its overall point about purposive construction. Insulet argues that there are similarities between this Patent and the patent which was considered in Catnic and that there is no plausible reason why the patentee would have intended it to be limited to a housing which is completely free of user input controls, excluding even basic safety mechanisms, and that construing the patent purposively requires it to be given a ‘more practical’ reading.
187. Roche replies that Catnic dealt with a very different situation, referring first to the points made above about ‘absolute’ and ‘relative’ terms, and secondly, noting that the patentee in this case has explained exactly why the housing should be free of user input components for providing flow instructions to the local processor – to deliver the advantages it describes and to seek to distinguish the claimed device from the prior art. Finally Roche notes that, if a stop button were desired, as Insulet suggests, the patentee has explained that a mechanical one can be used and the claims do not exclude that.
Construction of Integer 1G
188. I do not accept Insulet’s submissions that Integer 1G should be construed to cover devices having electromechanical user input components on the covering.
189. I have already set out above my view of the overall purpose of the patent. However, a patentee may have several purposes and there can be no presumption that the patentee necessarily intended the widest possible meaning consistent with his purpose to be given to the words he uses.
190. When looked at in the context of paragraph [0059] and the other paragraphs referred to above, the purpose of the patentee in drafting the language of the claim as he did was to achieve the benefits consistent with the overall purpose of the patent by the means identified in the integer and the limitation in the claim is consistent with that purpose.
191. The authorities relied on by Insulet to suggest that in this case the words “free of” user input components for providing flow instructions to the local processor should be read as encompassing a device on which the housing is “not free of …” do not support that position. I agree with Roche as to the guidance to be derived from paragraph [0114] and do not agree with the gloss suggested by Insulet. Insulet’s proposed reading is not to be preferred given the overall context of the specification and how that would be read by the skilled person. Having regard to the wider context of the Patent and the specification, the skilled person would understand that one purpose of the language used was to distinguish the device claimed by the Patent from existing devices and to achieve the benefits specified in, for example, paragraphs [0015] and [0059].
192. There was some dispute between the experts as to what the different costs of mechanical and electro-mechanical buttons would be and the importance of that to the patentee when drafting the claims. This is discussed further below in the context of infringement. Given the Patent’s repeated emphasis on cost and complexity, and the focus on the removal of electromechanical buttons to achieve that goal, part of the patentee’s concept (whether right or wrong in fact), seems to me to have been that the removal of electromechanical buttons with the associated circuitry was important to achieving the purpose of reducing cost and complexity, tending towards the realisation of a low cost, light weight device, capable of being disposable. Paragraph [0006] further supports such a reading.
193. Mr Causey’s acceptance that “… the teaching of the Patent is that it is the removal of all the electromechanical switches from the device that allows the electronics to be moved from the fluid delivery device to the remote control, and significant weight and volume to be removed from the fluid delivery device itself.”; his acceptance that one of the intentions of the patentee was to remove the ‘expensive electronics’ from the fluid delivery device; his agreement in cross-examination that the Figures 10 and 11 embodiments were mechanical user input components which do not provide flow instructions to the local processor; and his agreement that the claim allowed user input components on the housing, as long as they were not for providing flow instructions to the local processor, further suggests to me that that the skilled person would have considered the patentee to have concluded that achieving the purpose of the invention would require the removal of all electromechanical components from the housing.
194. Insulet suggested that a skilled reader would not necessarily have reached that conclusion from reviewing Figures 10 and 11 because it was clear from those figures that the system used in the figures was mechanical, rather than electromechanical, so nothing should be read into the fact that the buttons were mechanical. In the overall context of the Patent and the descriptions I do not agree.
195. While Claims 9 and 43 appear to envisage the delivery of some flow instructions directly from user input components which are not on the remote control, the fact that they are dependent claims means that they must be read to narrow the scope of Claim 1, rather than to expand it. In context, given what is said above about the overall purpose of the patentee, the skilled person would read those claims as permitting some limited input from mechanical user input components where used and no more broadly than that.
196. As to the final argument on Integer 1G, I prefer Roche’s submissions, in particular in the light of paragraph [0006] and the fact that alternative safety options are available, including the use of mechanical buttons and/or completely alternative means, such as the use of an injector pen and the removal of the device which were discussed by both experts and specifically referred to during the hearing. I do not consider this case to be similar in any material respect to Catnic on its facts.
197. In summary, therefore I construe Integer 1G as meaning that the housing must have no user input components which can provide flow instructions to the local processor of the device but may have components such as the mechanical stop and bolus buttons shown in Figures 10, 10a, 11 and 11a and described at paragraphs [0018] and [0088]-[0094].
“wherein the transcutaneous patient access tool is integrated into the exit port assembly” (all claims in issue - Integers 1H, 2I, 3I, 42A, 45B)
198. I have discussed above the construction of the terms EPA (see paragraphs 145-148) and TPAT (see paragraphs 149-150). The construction question which remains is the meaning of the word ‘integrated’ and how the three elements should be construed together in the light of the patentee’s overall purpose.
199. While the arguments and evidence on the construction of this integer were tightly bound into the arguments on validity and infringement, I understood neither expert to say that any part of it was a term of art and so the discussion on construction related to the purpose of the integer and the likely understanding of the skilled person.
200. Insulet states that the purpose of this integer is to distinguish the claimed invention from previous devices with an external tubing and infusion set. It contends that a key feature of the invention is that the EPA is connected to, and integrates, the TPAT and that this is a fundamental design change. Previous tubed insulin pumps required users to attach the end of the tubing distant from the infusion set to the reservoir. In contrast, the TPAT of the device in Claim 1 is “integrated into the exit port assembly”, which in turn is contained within the housing of the device. Insulet relies upon the evidence of Mr Causey as to how this integer would be understood by the skilled person.
“In the EP 764 device the TPAT is connected with and integrated into the exit port assembly. The two components will therefore connect directly to each other when the device is in use without any intermediate components. The exit port assembly must therefore be physically located immediately adjacent to the site of infusion i.e. the patient’s skin. In practical terms, this means that there is no tubing between the exit port assembly and the infusion site/TPAT and instead the pump is mounted on the skin of the patient, for example using an adhesive layer (see [0012] and [0078]-[0079]).”
202. In response to views expressed by Mr Treneman, he elaborates saying that “integrated into” does not require the TPAT to be fixed, or not detachable from, the EPA - rather that it requires only that the TPAT is directly connected to the EPA at the time of use. Insulet further submits that in that configuration there is no tubing between the EPA and the TPAT, leading to the tubeless, wearable pump which Insulet say is the invention claimed by the patent. During cross-examination, Mr Causey also noted that the tubeless aspect of the invention was supported in his view by various statements in the Patent that tubed embodiments were contrary to the invention which also tended to support his view as to the likely interpretation of ‘integrated’.
203. Roche submits that the Patent says little about the meaning of “integrated into” but that because paragraph [0055] states: “The exit port assembly 70 includes elements to transcutaneously enter the patient, such as a needle or soft cannula (not shown in Fig. 1)”, this suggests that the needle or cannula is a permanent part of the EPA, rather than detachable. Roche also argues that the fact that paragraph [0077] says that the device shown in Figures 5 and 5a “includes a transcutaneous patient access tool comprising transcutaneous micropenetrators 75 connected to the exit port assembly” does not suggest that the micropenetrators are somehow detachable from the exit port assembly.
204. Roche notes that the only point at which the description uses the word “integrated” in relation to the EPA is in paragraph [0110] which states that: “In the preferred embodiment of fluid delivery device 100, the transcutaneous delivery means are integrated into exit port assembly 70, however in an alternative embodiment, the exit port assembly can be attached to infusion set 400.” and disagrees with Mr Causey’s view that the distinction being drawn here is between:
“(A) an exit port assembly and a TPAT that at the time of use are directly connected with no intervening components such as tubing; and
(B) an exit port assembly and a TPAT that at the time of use are connected with an intermediate component like a length of tubing.”
205. Roche submits that Mr Causey’s emphasis on the situation at the time of use does not make sense in the context of paragraph [0110], which is concerned with the provision of components in kit form and that the distinction being drawn must relate to the device as provided to the user. Roche argues that the distinction is in fact between a transcutaneous delivery means which is an integral part of the exit port assembly and a case where such means can simply be attached to the exit port assembly.
206. Roche submits that this is consistent with paragraph [0104] (stating that what is contrary to the claimed invention is “an attachable transcutaneous infusion set”) and with the way in which the Patent deals with Figure 4. Paragraph [0073], which explains Figure 4, states that: “Contrary to the claimed invention, the fluid delivery device 10 of Fig. 4 also includes a Luer connector 71 for attaching a standard transcutaneous fluid delivery set to the exit port assembly 70.” This excludes the fluid delivery device of Figure 4 from the claimed invention, because it includes a Luer connector. Roche contends that the device is “adapted to connect to a TPAT” so it must be being excluded on the basis that it does not have the TPAT integrated into it and that “integrated into” must therefore mean that the TPAT must be fixed to, not just attachable to, the EPA.
207. Roche relies on the evidence of Mr Treneman that the Patent was distinguishing between a delivery means that is integrated into the device and not detachable, and a delivery means that is attachable to, and detachable from, the device.
208. In my view, Mr Treneman’s evidence on this point was tentative:
“Paragraph [0110] states that “…In the preferred embodiment of fluid delivery device 100, the transcutaneous delivery means are integrated into exit port assembly 70, however in an alternative embodiment, the exit port assembly 70 can be attached to infusion set 400….”. The Patent appears to be making a distinction between a delivery means that is integrated into the device and not detachable, and a delivery means that is attachable to the device (and detachable from the device). This leads me to believe that the Patent uses the term “integrated” to refer to a device with a fixed needle or cannula. This use of the term “integrated” accords with the use of the term in the following examples. An integrated circuit is an electronic device where all the discrete electronic components (transistors, resistors etc.) are within one unit and are sold and used as such. An integrated circuit is many components combined into one integrated assembly. A smart phone (such had just become available at the priority date of the Patent) had many different functions integrated into one product. Integrated is also used when a component is ‘built-in’ to a product at the time of supply and is not intended for removal by the user. An example of this would be the LCD screen on a 1999 mobile phone; it is replaceable if broken, but it is a separate component/sub assembly. Another example is a bonded vehicle windscreen. This is integrated in the sense that it is built-in and only replaceable using tools and equipment. However, I understand that the construction of the claims is a matter for the Court.”
209. Looking at the evidence in the round and having reviewed the Patent claims and the description overall I find the evidence given by Mr Causey more convincing on the meaning of ‘integrated’ in the overall context of the patent. Neither expert suggested that the word was a term of art. While I found Mr Treneman’s examples of the use of the word in other engineering contexts to be interesting, I do not consider that they mean that it is necessary to read into the Patent a requirement that the TPAT and EPA be inseparable, or essentially a single part. The use of ‘integrated’ in context carries the sense of combining or linking separate elements and I find that paragraph [0055] implies that the important time to consider the position is “when the local processor activates the dispenser …the exit port assembly includes elements to transcutaneously enter the patient, such as a need or soft cannula”. The description further notes that this configuration is not shown in Figure 1 which might also suggest that the important time for integration is at the time of use.
210. Considering paragraph [0073] of the description specifically, it states that “Contrary to the claimed invention, the fluid delivery device 10 of Fig. 4 also includes a Luer connector 71 for attaching a standard transcutaneous fluid delivery set to the exit port assembly 70”. This is in contra-distinction to the claimed invention when the EPA (which is common to all embodiments) can be combined with the TPAT which is thereby integrated with the EPA. I do not agree that Figures 4 or 5 bear the weight of interpretation placed on them by Roche; indeed paragraph [0077] refers to an embodiment in which the patentee is discussing the TPAT being connected to the EPA.
“Referring also to Fig. 5a, the device also includes a transcutaneous patient access tool comprising transcutaneous micropenetrators 75 connected to the exit port assembly 70. The transcutaneous micropenetrators 75 include a series of micro-needles or other micropenetrators that allow fluid to transcutaneously enter the body of the patient without standard needles. Similar transcutaneous micropenetrators are shown, for example, in U.S. Patent 5,983,136 to Karnen et al.”
211. On the other hand, paragraph [0080] mentions a needle insertion device and notes that it may be ‘integrated’ into the fluid delivery device or can be supplied as a separate mechanism, which might suggest that ‘integrated’ has a quality of permanence.
“Contrary to the claimed invention, a needle connection tubing 73 terminating in a skin penetrating cannula 72 is shown connected to the exit port assembly 70. The needle connection tubing 73 is flexible, allows various placements and can be reinforced to prevent kinking. Reinforcement can be accomplished through choice of materials and ratio of wall thickness to inner diameter, or the tubing 73 can be reinforced with an internal wire coil. The skin penetrating cannula 72 can be a rigid member, such as a needle, or can be flexible. The skin penetrating cannula 72 is inserted through the skin 210 prior to attaching the fluid delivery device 10 to the skin 210 and may be inserted using a needle insertion assistance mechanism. Such a needle insertion assistance mechanism may be integrated into the fluid delivery device 10, or can be supplied as a separate mechanism. Fig. 6 shows the cannula 72 entering through the surface of the skin 210 and entering subcutaneous tissue 211. Once the fluid delivery device 10 is attached to the skin 210, the needle connecting tube 73 remains relatively stable due to the direct connection between the device 10 and the skin 210. This stability helps prevent kinking of the tubing 73 and resultant occlusion, which is common to other ambulatory devices.”
212. In context, I do not consider, that this use of ‘integrated’ in a description of an element which is clearly an alternative means that it must be read in the same way when looking at the core description of the connection between the TPAT and EPA.
“a proximity alarm” (Claim 2 - Integer 2M)
214. The dispute surrounding the construction of this integer was tightly bound up with arguments on validity and it is more sensible to deal with it in that context.
“A kit containing a plurality of fluid delivery devices for delivering fluid to a patient and a single remote control device separate from the fluid delivery devices…” (Claim 3)
215. The dispute relating to this Claim was intertwined with the question of infringement and it is more efficient to deal with it in that context.
“wherein the local processor (50) is further programmed to provide flow information; and wherein the housing (20) is free of user output components for providing the flow information from the local processor to a user” (Claim 43, as dependent on Claim 42 - Integers 42B and 42C)
217. Roche argued that there is no basis for limiting “flow information” to information about fluid flow “which has taken place”, or to complex information about flow. Roche submitted that the patentee was excluding devices which contained any user output components on the fluid delivery device providing any information about fluid flow and that the Patent discloses no basis for limiting the plain meaning of the language used. Roche submitted that the Patent clearly contemplates users being provided with information by means such as lights, buzzers and vibration alarms (as well as LCD screens) on the remote control device and that the Patent acknowledges that the prior art devices included audio or vibration. Mr Treneman’s evidence was that he considered ‘flow information’ to include any type of information about insulin flows, including the use of auditory signals to confirm the receipt and execution of instructions, such as in respect of the delivery of a bolus.
218. Finally Roche referred back to its submissions on Integer 1G noting that paragraph [0059] applies to “user interfaces” in general, i.e. both user input components and user output components. Roche submits that the skilled person would understand Claim 43 to have a similar purpose and would read it accordingly.
219. Insulet also noted that “Similar points apply to those noted above in relation to integer 1G”. This appears to be a reference to the construction of ‘free of’.
220. While not fully developed in Insulet’s written submissions, Mr Causey’s evidence was that the patent would be understood as envisaging that the housing of the fluid delivery device could have means of communicating some information about flow to the user, as long as the information was not digital or complex in nature nor provided via an LCD screen. Mr Causey explained:
“flow information” is information provided by the local processor concerning the fluid flow which has taken place within the fluid delivery device and includes both basal and bolus insulin flow. In the EP 764 system this information can be transmitted to the remote control and can be displayed using user output components. See [0021].
221. Mr Causey subsequently explained that the main form of providing flow information to the user envisaged by the Patent is an LCD screen - although he does not exclude other types of information provision to users (such as through auditory cues) being described as ‘user output components’. Mr Causey notes, however, that in his view these are not user output components of the type envisaged by Claim 43 as they do not provide flow information to the user. Mr Causey appears to interpret ‘flow information’ as data, being essentially digital or electronic information about flow rates, rather than confirmatory information about the execution of commands.
222. During cross-examination of Mr Treneman, Mr Waugh QC referred to paragraph [0058] of the Patent as supporting Mr Causey. Given the time constraints, Mr Treneman was unable to express a view on whether that paragraph did suggest that flow information in the context of Claim 43 meant only complex flow information.
223. Paragraph [0058], referred to by Mr Waugh QC during his cross-examination of Mr Treneman, does not assist. That paragraph deals with the programming of the remote control to transmit complex flow algorithms and so on to the fluid delivery device. It says nothing about the meaning of ‘flow information’ as such but does envisage responses from the local processor of the fluid delivery device to the remote control device using electronic communication confirmation methods. Paragraph [0059] (discussed above) continues immediately after the discussion of the communication between the local processor and the remote controller and begins “The lack of user interfaces such as …, results in substantial reductions in the cost, the size and the weight of the device.” While it then goes on to mention the lack of information screens as an example of a user interface which is to be removed (as with the electromechanical buttons discussed above), it does not suggest that it is only such screens that must be removed, but rather that other user output components should also be removed. Unlike the discussion in relation to user input components above, there is no exemplary embodiment suggesting that user output components other than information screens on the housing of the fluid delivery device are envisaged, and paragraph [0096] of the Patent suggest that, like information screens more generally, such information sources are considered by the patentee as permitted only on the remote control device as alternative means for communicating with the user.
224. I conclude that ‘user output components’ includes any mechanism for providing information to the user and that Claim 43 requires that the housing must be free of all such components which provide “flow information from the local processor to a user”. I reach the same conclusion on construction here as in relation to Integer 1G. There is no reason to construe ‘free of’ in this claim any differently and it would be understood to mean ‘entirely without’.
225. The Patent gives little assistance on the meaning of “flow information”. The meaning has to be elicited from the overall context of the Patent and its goals. As discussed above, the overall purpose of the patentee is to reduce cost and complexity among other things so that the devices tend towards disposability. The removal of user output components is intended to achieve that purpose, as indicated by paragraph [0059]. In the absence of any indication from the language of the Patent that the patentee intended Claim 43 to permit some information about flow to be provided to the user by components on the housing of the fluid delivery device, I do not consider it justified to read a limitation into the language so as to construe ‘flow information’ to include only complex flow information, or data capable of being delivered digitally.
“for attachment to a skin surface of a patient” (Claim 45 - Integer 45C)
226. Roche submitted that this requires only that the device has to be suitable for attachment to a patient’s skin and cites various cases in support of that construction including Garmin (Europe) v Koninklijke Philips [2019] EWHC 107 (Ch) per Henry Carr J at [125]: “Normally, in a patent claim, where a device is required to be “for” a particular function, it means “suitable for” that function, and no more”. Roche further submitted that the Claim does not mean, for example, that the device has to be provided with adhesive. Insulet did not make separate submissions on the meaning of this claim but appeared to accept that the Patent requires that the fluid delivery device should be capable of attaching directly to the skin of the patient.
227. I agree that the natural reading of the language is to claim a fluid delivery device which is capable of attachment to the skin of a patient.
Infringement
The Solo Kit, Solo Components and Solo System
228. Insulet’s infringement claim relates to the Accu-Chek Solo system (“Solo”). That system has two main aspects: the Solo device or “micropump” which is a fluid delivery device and attaches to the user’s body; and the diabetes manager which is the separate remote control device. The infringement issues relate only to the Solo device.
229. Insulet provided a Product and Process Description (“PPD”) in lieu of disclosure, and the description below is based on that document and on the parties’ submissions. I understand it to be uncontroversial.
230. The Solo device is initially supplied as a starter kit which contains (among other things, not relevant to the infringement issues):
(i) two pump bases;
(ii) a remote control; and
(iii) an insertion device.
(i) a pump base;
(ii) a package containing eight reservoir assemblies;
(iii) a package containing thirteen infusion assemblies, each consisting of a cannula assembly and a pump holder; and
(iv) an insertion device.
232. The principal elements of the Solo device are:
(a) A pump base. This contains the transceiver chip and the microprocessor, among other things. It is recommended that it be replaced after four months. Diagrams of the pump base are below.

Front and rear of pump base (from Fig. 2 and Fig. 1 of PPD, respectively)
(b) A reservoir assembly. This contains a reservoir for insulin, a piston rod to engage with the motor in the pump base, and a flow path which terminates in a reservoir needle. It is recommended that it be replaced after up to four days of use. A diagram of the reservoir assembly is below.
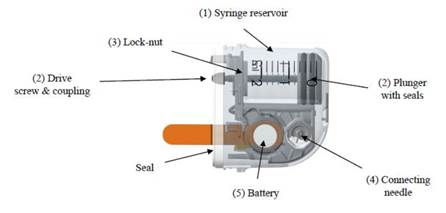
Reservoir Assembly (from Fig. 3A and Fig 3B of PPD respectively)
(c) An infusion assembly. This comprises the cannula assembly and the pump holder. Diagrams of the cannula assembly and pump holder are below. The pump is attached to the body via the pump holder and can be detached from and reattached to the body using attaching hooks. The pump holder is attached to, and the cannula inserted into, the patient’s skin using an insertion device. The infusion assembly is for single use only and is recommended to be replaced after up to three days of use. The insertion device can be re-used until it ceases to function owing to normal wear and tear, but is recommended to be replaced after approximately one year.
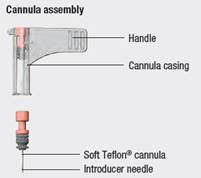
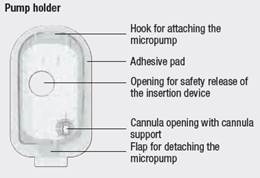
Cannula assembly and pump holder (from Fig. 4 & 5 of PPD)
233. Section 2.4 of the PPD explains how the Solo system is assembled and operated and is discussed as necessary below.
234. Section 3 of the PPD describes the user-controlled delivery of insulin. Basal insulin supply is controlled using the remote control. bolus insulin can be controlled using the remote control or by using the quick bolus buttons on the pump base.
235. Section 4 of the PPD describes user notifications, both those delivered via the remote control and those delivered via the fluid delivery device.
236. Further relevant features of the Solo system are addressed as necessary when considering the questions that arise on the issue of infringement.
The allegations of infringement
237. Insulet claims that Roche has infringed the Patent directly, by the manufacture and sale of kits containing the Solo device; and/or indirectly, by the supply of Solo Consumables.
Legal principles
238. Section 60 of the Patents Act is the relevant provision. So far as is relevant, it is reproduced below:
“(1) Subject to the provisions of this section, a person infringes a patent for an invention if, but only if, while the patent is in force, he does any of the following things in the United Kingdom in relation to the invention without the consent of the proprietor of the patent, that is to say—
(a) where the invention is a product, he makes, disposes of, offers to dispose of, uses or imports the product or keeps it whether for disposal or otherwise;
(b) where the invention is a process, he uses the process or he offers it for use in the United Kingdom when he knows, or it is obvious to a reasonable person in the circumstances, that its use there without the consent of the proprietor would be an infringement of the patent;
(c) where the invention is a process, he disposes of, offers to dispose of, uses or imports any product obtained directly by means of that process or keeps any such product whether for disposal or otherwise.
(2) Subject to the following provisions of this section, a person (other than the proprietor of the patent) also infringes a patent for an invention if, while the patent is in force and without the consent of the proprietor, he supplies or offers to supply in the United Kingdom a person other than a licensee or other person entitled to work the invention with any of the means, relating to an essential element of the invention, for putting the invention into effect when he knows, or it is obvious to a reasonable person in the circumstances, that those means are suitable for putting, and are intended to put, the invention into effect in the United Kingdom.
239. To succeed, Insulet must establish on the balance of probabilities that the product falls within the scope of protection of the Patent and that there has been an infringing act.
240. The correct approach to assessing infringement was set out in the Judgment of the Supreme Court in Actavis v Eli Lilly. That Judgment was considered and applied in Icescape. In the latter case at [66], Lord Kitchin (sitting in the Court of Appeal) explained that, following Actavis v Eli Lilly, two steps may be required when considering infringement:
· The first (and always necessary) step is to establish whether there is infringement of any of the claims as a matter of normal interpretation. Lord Kitchin confirmed that, when carrying out his exercise (and as previously noted by both Arnold J in Generics v Yeda and by Carr J in Illumina, Inc v Premaitha Health Plc [2017] EWHC 2930 (Pat)), the claims are to be construed purposively; the question is whether the product falls squarely within the wording of the claims as so interpreted. If infringement is established on that basis, nothing more is required.
· If infringement is not established on the ‘normal’ basis following step 1, there may be a need to consider whether the product nevertheless infringes because the way or ways in which it departs from the invention are immaterial.
241. As reiterated by Lord Kitchin, step 1 involves interpretation, while step 2 is a question which usually requires assessment of the facts and of the expert evidence.
242. To assist in carrying out the assessment required by the second step, Lord Neuberger considered in Actavis v Eli Lilly various authorities which had addressed when a variation from the language of the claims is or is not material and, in particular, the long established questions formulated by Lord Hoffmann in Improver Corporation v Remington Consumer Product Limited [1990] FSR 181. There is no need to set out all of Lord Neuberger’s reasoning here. It can be found at paragraphs [59] to [65] of his Judgment in Actavis v Eli Lilly and was helpfully discussed by Lord Kitchin at paragraphs [62] to [65] of Icescape. I have that reasoning firmly in mind below and refer to it as appropriate.
243. Following his thorough review of the approach required under Actavis v Eli Lilly, Lord Kitchin summarised the position as follows [66]:
“The whole approach to interpretation and scope of protection therefore involves the following steps, considered through the eyes of the notional addressee:
(i) Does the variant infringe any of the claims as a matter of normal interpretation?
(ii) If not, does the variant nevertheless infringe because it varies from the invention in a way or ways which is or are immaterial? This is to be determined by asking these three questions:
a) Notwithstanding that it is not within the literal (that is to say, I interpolate, normal) meaning of the relevant claim(s) of the patent, does the variant achieve substantially the same result in substantially the same way as the invention, i.e. the inventive concept revealed by the patent?
b) Would it be obvious to the person skilled in the art, reading the patent at the priority date, but knowing that the variant achieves substantially the same result as the invention, that it does so in substantially the same way as the invention?
c) Would such a reader of the patent have concluded that the patentee nonetheless intended that strict compliance with the literal meaning of the relevant claim(s) of the patent was an essential requirement of the invention?”
244. The immediately preceding paragraphs (62-64) are also of great assistance as Lord Kitchin explained that, when assessing the extent to which there is a material difference between the accused product and the invention, the Court must focus on ‘the problem underlying the invention’, ‘the inventive core’ or the ‘inventive concept’; and then consider whether, on being told what the accused product does, “the notional addressee would consider it obvious that it achieved substantially the same result in substantially the same way as the invention”. Lord Kitchin went on to explain that Lord Neuberger had made four points about how the third aspect of question (ii) should be addressed:
“i) Although “the language of the claim is important”, consideration of this question does not exclude the specification of the patent and all the knowledge and expertise which the notional addressee is assumed to have.
ii) The fact that the language of the claim does not on any sensible reading cover the variant is certainly not enough to justify holding that the patentee does not satisfy the third question.
iii) It is appropriate to ask whether the component at issue is an “essential” part of the invention, but that that is not the same thing as asking if it is an “essential” part of the overall product or process of which the inventive concept is part. Here regard must be had to the inventive concept or the inventive core of the patent.
iv) When one is considering a variant which would have been obvious at the date of infringement rather than at the priority date, it is necessary to imbue the notional addressee with rather more information than he might have had at the priority date. Here Lord Neuberger had in mind the assumption that the notional addressee knows that the variant works.”
245. The claims have been construed above in accordance with the purposive approach explained in Actavis v Eli Lilly and further elaborated by Lord Kitchin in IceScape. The first step is, therefore, to consider whether the Roche product falls squarely within the claims as so interpreted. If they do not, and for those where there is an arguable case that there may nevertheless be infringement by equivalence, the second step is necessary.
Claim 1
246. Only four integers remain in dispute: They are:
· Integer 1F (“a housing containing the exit port assembly, the dispenser, the local processor, and the wireless receiver”);
· Integer 1G (“wherein the housing is free of user input components for providing flow instructions to the local processor”);
· Integer 1H (“wherein the transcutaneous patient access tool is integrated into the exit port assembly”); and
· Integer 1I (“wherein the reservoir is contained in the housing and has a volume in the range of 2 to 3 ml”).
247. Each of these is dealt with separately below.
Integer 1F (“a housing containing the exit port assembly, the dispenser, the local processor, and the wireless receiver”)
Construction
248. This claim was construed at paragraphs 151-159 above as follows:
“The skilled person would understand the patent to claim a device with a covering which would serve the purposes of: containing the components safely and securely (including against foreseeable water ingress) in the normal course of a user’s daily life; maintaining a relatively smooth, rather than jagged or pitted, surface on which user interfaces had been reduced or eliminated, thereby assisting with cleaning; and enabling the device to meet its purposes of being low cost, light weight and potentially disposable. The language in the claims and description in context does not limit the claim to a device in which the covering is constructed and delivered to the user as a single contiguous part.”
Submissions on infringement
249. Insulet submits that the Solo device infringes because, when assembled, it has an outer surface which defines an exterior within which the exit port assembly, dispenser, local processor and wireless receiver are contained. Insulet submits that the views originally expressed in the written evidence of Mr Treneman about infringement proceeded principally from the starting point that the claim required a single housing as a matter of construction and are therefore fatally flawed.
250. Roche submits that there is no ‘housing’ containing all the required elements.
251. Roche also submits that the device does not fall within the claim because the device is not sufficiently resistant to foreseeable water ingress.
252. Mr Treneman stated that the Solo has an IP22 rating. He explained that IP (or Ingress Protection) rating is a standardised measurement (under, for example, EN60601-1) of the degree of protection offered by a device from among other things, water or dust. An IP22 rating means that the device is resistant to ingress from dripping water, if the device is held at an angle of 15 degrees. Mr Treneman explains that for a body worn pump to be showerproof would require an IP rating of IPX5 or more.
253. While Mr Causey said very little about water resistance in his written evidence, during cross‑examination, he agreed with Mr Treneman about the degree of water resistance claimed for the Solo, noting that in his view, there is no such thing as a water proof device. Mr Treneman contrasts the low IP rating of the Solo with that of some pumps that were available at the priority date of the Patent. He also noted that it is recommended that the Solo be removed before taking a shower or swimming.
254. After a protracted exchange during cross‑examination, Mr Waugh QC relied on Mr Treneman’s acceptance that, once assembled, the Solo device is water resistant and on the evidence of Mr Causey as demonstrating sufficient water resistance to meet the requirements of the claim.
255. On reviewing the transcript of Mr Treneman’s cross‑examination as a whole, it is clear that his view remained that the degree of water resistance claimed for the Solo was considerably lower than for other wearable devices and that the tests that it would pass under EN60601-1 would result in a much lower IP rating.
Assessment
256. I do not agree with Roche’s submission that as there is no “housing” containing all the elements that the claim requires the device falls outside the scope of the claim. On a purposive construction, the claim does not require that the device be constructed and delivered to the user with a housing which forms a single contiguous part. See also the discussion of infringement in the context of Integer 1H below.
257. The patent does, however, require that the device once assembled should have a covering which can contain the components safely and securely, including against foreseeable water ingress in the normal course of a user’s daily life. Given that the Patent’s purpose is to claim a device which is directly attached to the skin of the user, I conclude that the skilled user would be likely to consider that a product of this nature should have a significant degree of water resistance, enabling the user to wear it a least while showering. This is particularly the case in the light of the devices available on the market at the time. For example, both the MiniMed 508 and the DisetronicH-TRON plus had an IP rating of IPX7, denoting that a device is watertight against immersion up to 1m in depth for 30 minutes.
258. A level of water resistance to IPX7 might not be required to fall within the scope of the claim as the claim does not specify a particular requirement, and it appears that other levels of IP rating might be sufficient. However, the experts agreed that the way in which Roche’s product is designed provides only limited resistance to dripping water, and only at a particular angle. More importantly, the user is recommended to remove it when showering. It falls outside the claim on a normal construction.
Integer 1G (“wherein the housing is free of user input components for providing flow instructions to the local processor”)
Construction
259. As set out above, Integer 1G is to be construed as meaning that the housing must have no user input components which can provide flow instructions to the local processor of the device but may have components such as the mechanical stop and bolus buttons shown in Figures 10, 10a, 11 and 11a and described particularly at paragraphs [0088]-[0094].
Facts relevant to infringement
260. The principal facts relevant to the alleged infringement of this claim are:
· the Solo housing is free from user input components save for the quick bolus buttons. It is common ground that these are “user input components for providing flow instructions to the local processor”;
· these buttons can be seen in Figure 1 at paragraph 113. They are activated by the user pressing both buttons simultaneously and repeatedly;
· the purpose of these buttons is to provide instructions for the delivery of a bolus of insulin, without the use of the remote control device;
· they are an auxiliary means of delivering a bolus, described by Mr Causey as an “optional, limited and less preferred means of causing bolus volumes to be delivered”;
· the quick bolus buttons may be activated or deactivated using a setting in the remote controller. Mr Causey has stated that disabling the buttons would be strongly desirable for safety reasons;
· the quick bolus buttons cannot be used to programme the basal flow of insulin; the bolus increment (how much the insulin dose is increased with each button press); maximum bolus amount (overall cap on insulin delivery in a bolus); or delivery delay. All of these require the use of remote control.
Normal infringement
261. Insulet’s arguments on normal infringement were significantly intertwined with Insulet’s arguments on construction and rest largely on an interpretation of Integer 1G which has not been upheld and which has been dealt with above. Given the construction of the claim, most of those arguments are bound to fail on a normal approach to infringement, in the light of the acceptance by Insulet that the bolus buttons are user input components for providing flow instructions to the local processor and that they are clearly on the housing of the Solo device.
262. The only issue on normal infringement which arguably remains live is Insulet’s submission that, in the case where a user disables the quick bolus buttons using the Diabetes Manager, the result would be a device without any operable user input components on the housing as the buttons would be incapable of sending flow instructions to the local processor. In support of this factual position, Insulet refers first, to Mr Treneman’s acknowledgement, that when disabled the bolus buttons will not provide flow instructions to the local processor; and secondly, to evidence said by Insulet to indicate that the buttons are likely to be disabled for significant classes of user.
263. On this remaining issue, Roche’s position was that as a matter of fact the quick bolus functionality is enabled by default, although it can be disabled using an icon on the ‘bolus settings’ screen of the remote control. This was not disputed by Insulet.
264. Given that factual premise, Roche submitted that the authorities are clear that turning a device or function off does not render it unsuitable for its purpose, or mean that an apparatus will cease to infringe merely because it was switched off. Roche submitted that this is also true in reverse, that an apparatus which would not infringe when switched on will not suddenly become infringing when switched off.
265. Floyd J (as he then was) held in Qualcomm Incorporated v Nokia Corporation [2008] EWHC 329 (Pat) at [73]-[74]: “The question in each case is whether the apparatus, as it stands, is suitable for use in that way. If the apparatus has to undergo physical modification before it can be used, then prima facie it is not suitable for use and does not infringe”, a position which was reiterated by Arnold J (as he then was) in Parainen Pearl Shipping Ltd & Ors v Kristian Gerhard Jebsen Skipsrederi AS & Or [2018] EWHC 2628 (Pat) at [50].
266. I prefer the submissions of Roche on this point. In my view, the authorities are clear that if a device is capable of carrying out its function without adaptation or modification and is otherwise within the scope of the patent, it will infringe. Turning off a device does not change its function. Merely because the bolus buttons which take the Roche device outside the scope of the Claim on a normal approach to infringement can be turned off does not render the device infringing, any more than it would have rendered the device non-infringing in other circumstances. The quick bolus buttons are still for providing flow instructions to the local processor, even when the functionality has been disabled; no ‘modification’ or ‘adaptation’ is required and the device therefore falls outside the scope of the claim on a normal approach to infringement.
Infringement by equivalents
267. Insulet submits that, insofar as the Solo device does not fall within Integer 1G on a normal interpretation owing to the existence of the quick bolus buttons, the presence of the quick bolus buttons is an immaterial variant and the device is within the scope of Claim 1 under the doctrine of equivalents as it is a small, low cost, light weight, “patch” (or wearable) pump which is operable via a remote control.
268. The approach to be taken to such arguments following the Supreme Court’s Judgment in Actavis v Eli Lilly has been discussed above. The submissions of the parties are summarised briefly below.
- First Actavis question: is substantially the same result achieved in substantially the same way as the invention, i.e. the inventive concept revealed by the patent?”
269. The parties had quite different views on the relevant ‘inventive concept’.
270. Insulet’s skeleton argument summarised Insulet’s contention that that the inventive concept of the patent is a small, low cost, light-weight “patch pump” which is operable via a remote control and further submitted that Integer 1G seeks to contribute to achieving this outcome by relocating substantially all of the user input components to the remote control, so as to make the pump smaller, lighter and cheaper. The more expensive components can then be replaced less frequently or not at all, since they are situated on the remote control rather than on the pump. The resulting pump housing is thereby miniaturised, easier to produce, more durable and has a smoother outer surface. Insulet did not regard the removal of substantially all of the user input components from the housing as part of the inventive concept.
“The Claimant will contend that the inventive concept of EP764 is a small, low-cost, lightweight, simple to use, programmable and adjustable ambulatory device, and system for patient infusion, having the features claimed which overcomes the problems outlined in paragraph [0007] of the Patent.”
272. Roche contended in its skeleton argument that:
“The inventive concept of the Patent is a remote-controlled ambulatory infusion pump (with a housing containing a wireless receiver, a processor, a reservoir, a dispenser and an exit port assembly which can connect to a transcutaneous delivery means) which has no electromechanical controls on the housing. It is the removal of all electromechanical controls from the housing which allows the device to be made smaller, lighter, less complex, cheaper, and hence disposable.”
273. Roche submitted that the specific teaching of Integer 1G was a core part of the inventive concept, being a feature mentioned repeatedly as giving rise to the advantages identified.
274. Roche submits that the inventive concept for the purposes of identifying infringement by equivalents must be identified at the right level of generality, referring to the comments of Mellor J in Mitsubishi Electric Corporation v Oneplus Technology (Shenzhen) Co, Ltd & Ors [2021] EWHC 1048 (Pat), at [147].
Assessment
275. I have discussed the patentee’s purpose above. The views I expressed there when construing this claim are very close to the inventive concept contended for by Insulet in its RFI Response. I consider that Insulet’s description of the inventive concept in that pleading to be an appropriate starting point for the consideration of the first Actavis question. However, it is a starting point only. It is necessary to ask whether this description of the inventive concept encompasses only the matters referred to by Kitchin LJ in IceScape as “the problem underlying the invention and the inventive core”, while also bearing in mind the comments of Floyd LJ in the same case about the continuing importance of the claims.
276. Mr Tappin QC, for Roche, submitted that in cases such as Ice Scape, Akebia and Actavis v Eli Lilly where infringement by equivalents was seriously entertained, the alleged infringement differed from the patent claims only in ways which had nothing to do with the problem underlying the invention or the inventive core. In his view, this suggested that the ‘inventive concept’ to be protected under Actavis v Eli Lilly would encompass the matters specifically claimed and would be ‘firmly rooted in the Claims’.
277. Insulet’s pleading on this issue (quoted at paragraph 271 above) was provided before the expert evidence although, given the nature of the response, it does not seem to me that anything turns on that for these purposes. Insulet initially adopted a similar approach to that of Roche when stating the inventive concept for which it would contend, referring specifically to a device and system having the features claimed. However, Mr Waugh QC’s written submissions omitted that language and criticised Roche’s contention that the Solo quick bolus variant is “directly contrary to the inventive concept of keeping the housing free of user input components”, contending. that Roche had erred by confusing means and result.
278. In essence, the dispute can be boiled down to the question whether, when identifying the inventive concept for the purposes of assessing infringement, it is sufficient simply to consider the overall result to be achieved or whether the means used to achieve the result are also part of the inventive concept.
279. In many, perhaps most, cases this may not matter in practice and, given what I say below, ultimately it may not matter in this case. However, if a conclusion on this issue becomes necessary, I conclude that the first part of the second Actavis question requires the identification of the inventive concept not at a high or generalised level, but having regard to means identified in the patent to achieve the result desired by the patentee. This flows from the language used by Kitchen LJ in Ice Scape where the phrase “inventive concept” follows and to my mind encompasses not only the result which is the overall subject of the patent but also to the means by which that result is to be achieved. As Kitchin LJ explained at paragraph 62 of his judgment in IceScape, considering the guidance given by Lord Neuberger in Actavis v Eli Lilly:
“He thought … the Court must focus on ‘the problem underlying the invention’, ‘the inventive core’, or ‘the inventive concept’. In effect, the question is whether the variant achieves the same result in substantially the same way as the invention.” (Emphasis added).
280. In other words, the result and the means used to achieve it are all part of the inventive concept and it is on this that the Court must focus when considering infringement.
281. This focus on the claim language is also reflected in the brief judgment of Floyd LJ in IceScape commenting (among other things) on the correct approach following Actavis v Eli Lilly:
“… There is a second, non-interpretative exercise which allows the patentee a degree of protection outside the normal, purposive meaning of the claims where the variant from the claim achieves substantially the same effect in substantially the same way.” [96]
“It should not be thought, however, that the claims do not continue to have an important function. It is variants from the claim which have to achieve substantially the same effect in substantially same way as the invention. The claims remain the starting point for the subsequent analysis of variants. Although we may have edged closer to it, the new approach does not transgress the second of the outlawed approaches in the Protocol, which treats the claim merely as a somewhat vague guideline.” [97]
Submissions
282. Both parties accepted that a critical aspect of the question as to infringement of Integer 1G by equivalents was whether substantially the same means were being adopted by the allegedly infringing device and the Patent.
283. Insulet first submits that if it is accepted that mechanical buttons would be permitted by the patent, then the first Actavis question must be answered positively as, in Insulet’s submission, both mechanical and electromechanical buttons achieve substantially the same result by substantially the same means:
“… the actuation of user input to deliver a bolus of insulin is the result, and the means is a button pressed by the user. The electromechanical variant still achieves the objectives sought to be attained by the invention, since its marginal cost is insignificant, it does not materially increase the size or weight of the device, and in a pump which already has a processor and associated electronics (as taught at [0054] of the Patent) is immaterial to its overall complexity and disposability, nor does it detract in any way from the tubeless/patch concept.”
284. Insulet’s position is that the Solo system embodies the Patent’s inventive concept, and achieves substantially the same result in substantially the same way because: it is a tubeless ‘patch pump’; controlled via a remote control; with a relatively disposable, light-weight, small and simple fluid delivery device. Like the mechanical button embodiment foreseen by the Patent, the device has emergency bolus buttons that can be pressed by the user, while the housing is substantially free of user input components, save for the limited safety backup controls of a kind that the Patent teaches might be retained on the housing. While these are implemented electromechanically rather than mechanically, this is said not to be a material distinction.
285. Insulet refers to the factual context and notes, in summary:
(a) almost all the complex elements are situated in the remote control;
(b) the buttons are subordinate, play a back up role and can be de-activated;
(c) the buttons are flush against the housing, maintaining a smooth outer surface and the Solo is small, lightweight and sufficiently cheap to be disposable at quarterly intervals;
(d) the Solo is tubeless owing to the integration of the TPAT with the EPA - this is said to be one of the key inventive advances of Claim 1; and
(e) the quick bolus buttons do not add materially to the weight, bulk or cost.
286. Roche submits that, even if keeping the housing free of electromechanical controls is not part of the inventive concept itself, the Solo does not achieve substantially the same result in substantially the same way. Roche disagrees with the factual points made by Insulet on the basis that they are irrelevant (e.g. that the buttons can be de-activated); wrong (as a matter of principle because they disregard the claimed feature altogether by focussing on other aspects of the invention - e.g. the focus on tubelessness); or inaccurate (as a matter of fact because on the evidence the quick bolus buttons do add materially to cost and size and undermine disposability).
287. Roche’s position is that the buttons differ materially from the teaching of the Patent and submits that the Patent’s aim of delivering a “small, light weight and low cost” device is taught as being brought about by ensuring that “the housing is free of user input components for providing flow instructions to the local processor”. Roche notes that Mr Causey agreed that “Moving user input components for providing flow instructions to the local processor onto a separate remote control is an important part of the way in which the inventive concept of EP 764 is achieved” and submits that the quick bolus buttons do not achieve substantially the same result in the same way because they:
(a) provide flow instructions to the local processor;
(b) take up space on the device, and the electronic components associated with their functioning take up space within the device, both factors limiting the extent to which the size of the device can be reduced;
(c) impose additional manufacturing costs; and
(d) do not give rise to a device that is low enough in cost to be disposable in the sense described in paragraph [0014], i.e. as frequently as every two to five days (i.e. when the infusion site needs to be changed). Instead the Solo’s pump base is retained for up to four months.
Assessment
288. The key is the extent to which including two electro-mechanical buttons on the outer housing of the device deviate substantially from the scope of the invention claimed by the Patent because they result in:
· greater size, thus affecting ‘wearability’; and/or
· greater bulk, affecting wearability and smoothness; and/or
· greater cost, affecting disposability.
289. As submitted by Insulet, these questions are to be assessed by reference to the embodiment involving the addition of some mechanical buttons.
290. As foreseen in IceScape, this assessment moves beyond interpretation and requires consideration of the evidence. There are significant disputes between the experts on each issue. As might perhaps be expected, overall Mr Treneman’s evidence tends to establish that the degree of added cost and bulk resulting from the addition of electro-mechanical rather than mechanical buttons would be sufficiently significant to affect the device’s ability to achieve substantially the same result as that foreseen by the patent, while Mr Causey’s evidence tends to the opposite conclusion. There is little evidence other than that given by the experts. In assessing the value of that evidence I bear in mind that neither devoted a significant portion of his report to these issues and it does not appear that either undertook any empirical work.
291. Mr Causey’s evidence was that the quick bolus buttons do not add materially to the weight, size, bulk, or cost of the infusion device. This view was expressed briefly in one paragraph of his first report:
“The Quick bolus Buttons do not protrude from the profile of the micropump and I do not expect they would add significant weight, volume or cost to the micropump. I consider that the Solo System embodies the inventive concept of EP 764 and achieves the same result irrespective of the addition of the Quick bolus Buttons. In relation to this feature, the same result is achieved by removing the bulky, heavy and expensive electronic user interface components from the fluid delivery device and locating them on a separate remote control (the Diabetes Manager). The Solo device also incorporates an exit port assembly integrated directly into the TPAT into the Solo device such that it is worn on the skin. This is the same means of achieving that as in EP 764.”
292. During his cross‑examination, commenting on the evidence given by Mr Treneman, Mr Causey further explained that he did not consider that cost would be a serious consideration at all and indicated that any buttons could be quite small:
“A. As to the cost, it would be insignificant, I believe. The cost of plastic components would be in the pennies. If the buttons are co-moulded with the case it would again be a very low-cost addition, so I do not believe the cost is a serious consideration at all and ----
Q. Okay. But that is your ----
A. ---- possibly in my opinion that the buttons could actually be quite small.
Q. Right, but looking at the Solo, he is right, is he not, that they require electronic components to remain on the device in order to facilitate their operation; correct?
A. The electronic components are simply circuit board traces. You cannot put traces on a plastic circuit board, so with the local processor already there, there is almost nothing additional.”.
293. Mr Treneman’s written evidence on cost, bulk and disposability was, in summary, that the quick bolus buttons require electronic components to remain on the device, take up some space on the device and protruded from the device. He was very clear that the quick bolus buttons would add cost. His view was that additional costs would arise from the addition of the buttons themselves, noting that PCB mounted switches such as those on the Solo device were more expensive than alternative silicone rubber switches. He also expected significant additional costs to arise because the process required to manufacture the buttons for addition to the casing of the pump base “would require complex and expensive tooling to insert the quick bolus buttons into the casing”.
294. Mr Treneman also commented on the disposability of the device, noting that:
“I consider that the cost of the pump base is reflected in the fact that the pump base is durable and reusable, and is only required to be replaced after up to four months. The pump base is not disposable in the sense that the pump holder, cannula and the reservoir assembly are disposable, these components being replaced every few days. Nor is the pump base disposable in the sense described in the Patent, where it is said in paragraph [0014] that “Aspects of the present invention will enable cost reductions significant enough to make the entire device disposable in nature, being replaced as frequently as every two to five days. A disposable device allows the medication to be prefilled by the manufacturer and does not need the routine cleaning and maintenance required by long term devices, greatly simplifying use for the patient.””
295. Mr Treneman was robustly cross-examined on this aspect of his evidence. He explained the consequences of the use of PCB mounted electromechanical switches first for broader design considerations; and then for costs more generally:
“One of the difficulties of those buttons in the bigger picture is that they have to have microswitches on the inside that you press. They have to be mounted and connected. There has to be more processor capacity on the inside. There has to be software to deal with it, and then there have to be outputs from the software in order to confirm to the patient what bolus they have asked for and when it is delivered, when it is starting, when it is stopping and so forth. So, just putting the buttons on is not really the focus; it is all the other engineering behind it, as somebody in my position would know.”
“I am a mechanical engineer. I have commissioned probably 500 tools, some of them two-shot tools. I know what I am talking about in terms of the cost of a two-shot tool. They are very, very difficult to do. The tooling is more expensive and there are reliability issues. It is not like putting a piece of soft rubber around a toothbrush, it is not like that. Inside of the Solo device, there are electromechanical switches, physical switches that you can feel when you press it. If you got hold of the device and pressed the grey buttons you can feel them clicking. So they are not silicone, carbon pill, they are PCB-mounted switches, and then that means that you have to worry about switch balance [bounce][1] for the information and also you have to press two of them together, which means that there has to be a timing issue. The software has to be looking at both switch inputs and they have to be pressed within a certain timing input and it is quite complex. It is not just a small piece of work to do that. And that I do know.”
296. Mr Treneman was very clear that, in his view, Mr Waugh QC (and Mr Causey) were wrong to suggest that simply because the devices themselves cost ‘thousands of dollars’ the additional costs involved in adding electromechanical switches was ‘relatively trivial’. He noted that the important factor was to consider the additional impact on the bill of materials rather than drawing a comparison between the additional costs of particular components and the end sales price.
297. Considering the evidence in the round, there was no meaningful evidence that the bolus buttons added significantly to the weight or size of the device. Mr Causey was of the view that they did not, and Mr Treneman’s evidence was tentative at best. Although Mr Treneman stated that the buttons would take up space and protrude he did not attempt to explain how these factors would affect any material aspect of the invention. I have already concluded above in the context of the debate on the construction of Integers 1F and 1G that the key considerations underpinning both Integers are the concerns about wearability and disposability. The device remains sufficiently small and light to be wearable, and the fact that the buttons might protrude does not in my view go to the inventive concept as the patent requires the outer housing to be only “relatively smooth” (paragraph [0059]).
298. The situation in respect of costs is different. I do not accept Mr Waugh QC’s submission that “the evidence of Mr Causey was clear, based on his direct knowledge and experience, and supported by clear explanations” and therefore “clearly to be preferred to that of Mr Treneman, who offered little more than bare assertion not backed by any relevant experience”. Neither expert had carried out an analysis of the additional costs involved in adding quick bolus buttons of the type used in the Solo device, either as against a device with no buttons or a device with only mechanical buttons. Mr Causey barely touched on the issue of cost or its implications in his written report, and his evidence during cross-examination was neither detailed nor supported by any background assessment. As Mr Waugh QC rightly pointed out, Mr Treneman had not done any detailed costs analysis either. However, he had clearly turned his mind to the issue of costs, how they might occur, where they might fall and what the impact might be. On this issue, I therefore prefer the evidence of Mr Treneman that the addition of the electromechanical bolus buttons will add costs to a non-trivial extent.
299. One of the key aspects of the inventive concept is that the device lends itself to being small (thus wearable) and disposable in nature. Both parties agreed that the reduction in costs achieved by removing the expensive electronics to the remote control device was a key aspect of achieving that goal. The patent envisaged that as a result the device covered by the invention would be low enough in cost to be disposable as explained at paragraph [0014] of the description (i.e. every two to five days). By contrast, the Solo device continues to have electromechanical components on its housing which the evidence suggests increase cost and complexity and the device as a whole is not sufficiently cheap or lacking in complexity to be disposable in substantially the way envisaged by the patent (see the description of the various elements of the overall device and their variable disposability at paragraph 231 above). In the light of the above, I conclude that the answer to the first Actavis question is that the Solo device does not achieve substantially the same result as the invention in substantially the same way.
300. Given the conclusion on the first Actavis question, it is not necessary to answer either the second or third Actavis question. In any event, Roche accepted that if the answer to the first Actavis question was ‘yes’ then the answer to the second Actavis question should also be yes. In the event that it may be of future relevance for my view on the third Actavis question to be clear, I deal with it briefly below.
- Third Actavis question: Would the skilled person conclude that the patentee intended that strict compliance with the literal meaning of the claim was an essential requirement of the invention
301. At [65] of his judgment in Actavis v Eli Lilly, Lord Neuberger indicated that the underlying thrust of this question was whether the feature in issue was an essential part of the invention, in other words whether it would have been regarded by the skilled person as essential to the inventive concept, or inventive core, of the patent.
302. Roche submitted that in the present case, the skilled person would have regarded the feature as essential to the inventive concept as this is not a case where a term appears in a claim without any emphasis in the description as to its importance to the invention. Roche contended that the description in the Patent repeatedly emphasises the importance to achieving the patentee’s objectives of making the housing free of user input components for providing flow instructions to the local processor. Had it been unimportant, the patentee could have stated that the housing should be substantially free of such user input components (or something similar) but did not. Roche relied on the significance to the third question of a clear teaching in the description as noted by Birss J (as he then was) in Illumina Cambridge Ltd v Latvia MGI Tech SIA & Ors [2021] EWHC 57 (Pat) at paragraph 396 finding the answer to Actavis question 3 to be ‘yes’ in that case: “The reason why skilled person would think that strict compliance with the normal construction of “incorporation” was essential is because the specification has gone out of its way to define that term in a clear and simple way. It is not necessary for the skilled person to speculate about why the patentee may have done that, the fact is that it has been done.”
303. Roche submitted that the fact that the Patent permits the inclusion on the housing of user input components which do not provide flow instructions to the local processor, and describes and illustrates mechanical stop and bolus buttons, is a further point in its favour as the patentee has clearly considered that stop and bolus buttons on the housing may be thought desirable, but has specified that they should not provide flow instructions to the local processor. For the skilled person this would also fit with the fact that this teaching was an attempt to distinguish the invention from the prior art.
304. Finally, Roche contended that the answer ‘yes’ to the third question is consistent with the Protocol on the Interpretation of Article 69 of the European Patent Convention while extending protection as contended for by Insulet would go beyond fair protection for the patentee and would not afford a reasonable degree of legal certainty for third parties.
305. Insulet disagreed, largely for reasons similar to those it put forward in relation to purposive interpretation, and those reasons have been dealt with above in that context.
306. In conclusion, it is my view that Insulet has not established that the third Actavis question should be answered negatively. In my view, the skilled addressee would conclude that the patentee did intend there to be strict compliance with Integer 1G.
307. In summary therefore, the answers to the first two Actavis questions are no. However, in the event that I am wrong about that and that the first and second Actavis questions are to be answered yes, I consider that the third Actavis question should also be answered yes, and the Solo device does not infringe Integer 1G by equivalents.
Integer 1H: “… wherein the transcutaneous patient access tool is integrated into the exit port assembly …”
308. This integer has been construed above. For convenience, the construction of the relevant constituents of the integer are set out below.
· an EPA is a means of connecting the flow path from the reservoir to the flow path through the TPAT;
· a TPAT is an element that pierces the skin and enables continued transcutaneous infusion of insulin such as a needle cannula or array of microneedles;
· ‘integrated’ in context carries the sense of combining separate elements as part of a system. The claim does not require that the TPAT be fixed to the EPA at all times or delivered to the user in that configuration: the claim requires integration between the TPAT and the EPA at the time of use;
· the integer as a whole claims a device in which, at the time of use, two elements (described as the EPA and TPAT) combine together to allow fluid to flow between them and directly through the transcutaneous element into the user.
Infringement facts
309. The Solo delivers insulin via a sterile soft Teflon cannula. The PPD explains that the Solo micropump is connected to the body using the infusion assembly. This involves the cannula assembly, the pump holder and the insertion device. The relevant figures are reproduced below.
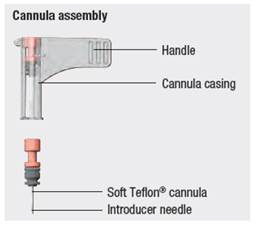
Figure 4
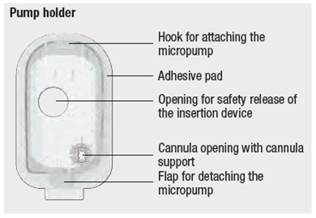
Figure 5
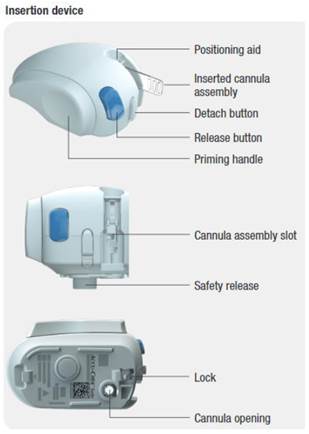
Figure 6
310. The PPD explains that the pump holder affixes and supports the cannula in place for the subcutaneous delivery of insulin via the cannula support. When the micropump assembly is connected to the pump holder, the reservoir needle pierces the cannula septum, and the end of the reservoir needle sits within the cannula head. Figure 13 of the PPD contains cross-sections showing the relevant parts of the Solo and the pump holder with the cannula inserted into the patient’s skin, before and after the micropump assembly is connected to the pump holder. These drawings are reproduced below.
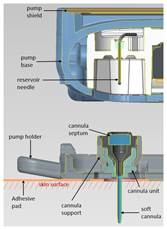
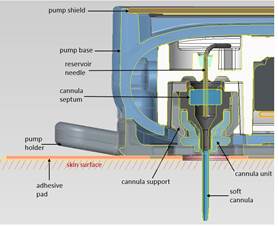
311. The experts broadly agreed on the way in which the Solo functions.
Infringement arguments and evidence
312. Insulet contended that the Solo clearly falls within Integer 1H as the Solo does not utilise external tubing; it has a TPAT in the form of a soft cannula made from Teflon, which is inserted into the patient’s skin using an insertion tool and attaches directly to the fluid delivery device so that when assembled for use, the TPAT is integrated into the Solo and forms a sealed fluid delivery path.
313. Insulet relied on the evidence of Mr Causey that:
“In the EP 764 device the TPAT is connected with and integrated into the exit port assembly. The two components will therefore connect directly to each other when the device is in use without any intermediate components. The exit port assembly must therefore be physically located immediately adjacent to the site of infusion i.e. the patient’s skin. In practical terms, this means that there is no tubing between the exit port assembly and the infusion site/TPAT and instead the pump is mounted on the skin of the patient, for example using an adhesive layer (see [0012] and [0078]-[0079]).”
314. Insulet submitted that the purpose of this integer is to distinguish the claimed invention from previous devices with an external tubing and infusion set and that the Solo clearly fell within the claim.
315. Roche’s first argument on infringement rested on the meaning of ‘integrated’. Given the way in which the claim is to be construed, that argument cannot succeed and is not considered further.
316. Roche’s second argument on infringement was that there is no direct connection between the cannula and the reservoir needle in the Solo system as for insulin to reach the cannula from the reservoir needle it has to pass through the cannula head. Roche submitted that, in effect, the cannula head acts as tubing providing a fluid path between the reservoir needle and the cannula meaning that it falls outside the scope of the claim.
317. Roche relied on the evidence of Mr Treneman in support of this argument. Mr Treneman explained in his second report that the cannula assembly of the Solo has multiple parts and that once the cannula has been inserted, there is a subcutaneous portion (which Mr Treneman refers to as the cannula) while the cannula head is proud of the skin and sits within the cannula support in the pump holder. He concludes that:
“… On assembly of the micropump and the pump holder, the reservoir needle pierces the septum within the cannula head. When fluid delivery to the patient is initiated, insulin flows from the reservoir needle, into the cannula head and then into the cannula itself. I have shown the flow of insulin cross-hatched in orange in the right-hand panel of Figure 9 below. There is no direct connection between the reservoir needle and the cannula itself.”
318. Mr Treneman noted that when connected, the “reservoir needle physically aligns with the cannula head” and that this allows a flow of liquid into the patient.
319. This issue was further explored during cross‑examination of Mr Treneman. He maintained that the soft cannula and the cannula head were separate components within the overall cannula assembly, that the TPAT should be construed as covering a needle or a cannula, rather than a cannula assembly, and that the cannula head was interposed between the TPAT and the EPA. He also stated that the important issue is the fluid path.
320. Finally, Roche submitted that during cross‑examination Mr Causey had given evidence about which elements of the integrated TPAT/EPA feature of the Solo formed part of the EPA and which formed part of the TPAT. It stated that some of the elements which Mr Causey had stated were part of the EPA were to be found on the pump holder and not part of the pump base. It submitted that Insulet had previously argued that the ‘housing’ of the Solo device comprised the pump base and reservoir, but not the pump holder and that therefore as Integer 1F required the EPA to be contained within the housing, there could be no infringement.
Assessment
321. As mentioned above, Roche’s first argument must fail.
322. Roche’s second argument relies on a drawing a distinction between the subcutaneous portion of the cannula (or cannula assembly) and that which sits proud of the skin, identifying the subcutaneous portion only as the TPAT and treating the cannula head as a piece of tubing, or at any rate as an intervening element which means that the TPAT is not connected to, and therefore not integrated with, the EPA.
323. Mr Waugh QC submitted that such an approach was plainly wrong, that there is nothing in the claim language to support such a narrow reading of TPAT and no reason to believe that the skilled person would approach it as requiring an atomisation of the cannula, and the exclusion of those portions above the skin. He submitted that what matters is that when in use there is a direct connection between the TPAT and the EPA so as to form a liquid seal. I agree with Mr Waugh QC. This is an area in which I found Mr Treneman’s evidence to be less than helpful, focussing on very small distinctions, and given with an eye to the infringement argument.
324. The claim is broad and, as noted by both experts, lacks clear definitions of the concepts it utilises. When purposively construed, there is no basis for reading limitations into the language in the way contended for by Roche. On that normal construction of the patent, I find that Roche’s second argument fails.
325. Roche’s third argument relies on a precise definition of EPA and the argument that, as a consequence, because the EPA (as so defined) is on the pumpholder, rather than the micro pump itself, it is not contained within the housing of the micropump, as required by Integer 1F.
326. Mr Waugh QC submits that this is not correct on various bases, which I can broadly summarise as being that on a normal interpretation of the claim, rather than one which relies on undue textual analysis, the Solo clearly infringes because what matters is that when in use there is an integrated means of fluid delivery to the patient, which does not require external tubing, and that when in use the device encompasses the means of integrating the flow path from the reservoir to the flow path through the TPAT.
327. I agree with Mr Waugh QC. I do not accept that a textual deconstruction of the expert evidence as to the precise configuration of the elements of the EPA is the appropriate approach to infringement given the normal construction of the claim set out above. Moreover, having reviewed Mr Causey’s evidence as to the meaning of ‘housing’ in respect of the Solo, he clearly included the pump holder:
“I have been asked to identify the “housing” in the Solo device. I consider the Solo has a housing when the components are joined and ready for its intended use (to deliver insulin to the user). The Solo device, when ready to be used, is an integrated assembly of the micropump (which itself is formed by the attachment of the pump base and filled reservoir) and the pump holder, at which stage the cannula is integrated into the exit port of the micropump. These parts are clearly designed to fit together and operate as a single integrated unit. The housing is the integrated external shell of the assembled device which encloses the internal components of the device.”
328. The evidence as to the operation of the Solo establishes that on a normal construction of the claim, the Solo falls within the scope of the language in Integer 1H. It is a device in which, at the time of use, two elements (described as the EPA and TPAT) combine together to allow fluid to flow between them and directly through the transcutaneous element into the user. I do not accept that the concepts of EPA and TPAT must be read so narrowly as to mean that the device is not within the scope of Integer 1H merely because some elements are to be found on the pump holder rather than the pump base when the device is not fully assembled.
Integer 1I: “… and wherein the reservoir (30) is contained in the housing and has a volume in the range of 2 to 3 ml”
329. The Solo contains a reservoir with a maximum capacity of 2.0 ml. The only dispute about this integer was whether it was within the ‘housing’ as required by Integer 1F. Given the construction of that integer, this aspect of the Solo falls within the claims.
330. Summary on Claim 1 - infringement:
· Roche accepts that Integers 1A to 1E are present in the Solo device;
· Integer 1F is not present in the Solo device because the housing once assembled does not have the characteristics required by this integer, in particular, as it is not sufficiently water resistant to be worn during daily life by the user;
· Integer 1G is not present in the Solo device. It does not infringe this aspect of the claim either on a normal basis or by equivalents. Having construed the Claim, the device does not fall squarely within it, nor does it achieve substantially the same result by substantially the same means;
· Integer 1H is present in the device;
· Integer 1I is present in the device.
331. In summary, owing to the absence of two important aspects of the claim, Claim 1 of EP ‘764 is not infringed by the Solo device.
Claim 2
332. There were no additional disputes relating to the requirements of Claim 2 as for the purposes of infringement they largely correspond to Claim 1.
Claim 3
333. This claim corresponds to original Claim 40 and claims a kit comprising a single remote control device including a plurality of fluid delivery devices.
334. The infringement dispute relating to Claim 3 arose in the context of indirect infringement.
335. Insulet submitted that the Solo kit includes multiple fluid delivery devices and a single remote controller which is a separate non-disposable device. It also submitted that it is obvious that the user will also receive an initial supply of reservoirs and infusion assemblies (i.e. pump holders and cannulas). There was no dispute that all of these elements were supplied and marketed by Roche. It therefore followed that Roche was supplying a system within the scope of Claim 3 and that it was irrelevant that users would not in fact assemble more than one device at a time. Insulet submitted that Roche could not import a limitation into the claim to require a plurality of fluid delivery devices to be fully assembled for delivering insulin at the same time. In Insulet’s submission, the skilled person would understand that a patient could only ever use one device at a time. There would be no reason to suppose that the patentee required them to be supplied with all the kit of devices in fully assembled form, or that the user would make all the devices ready for use in parallel.
336. Roche’s position was that there is nothing in the evidence that suggests that it knows (or that it is obvious) that users will use the components to assemble “a plurality of fluid delivery devices for delivering fluid to a patient” and, moreover, that there is nothing to indicate that users will in fact assemble more than one fluid delivery device at any one time. It points to the fact that the instructions are to use one pump base with the reservoir assemblies and infusion assemblies, disposing of the latter after four or three days respectively. Then, after four months, the pump base is disposed of and the other pump base is used. Replacement pump bases can then be obtained.
337. Roche had a further point that it would need to foresee that more than one device would be attached at the same time in order to fall within the scope of Claim 3 and noted that there is nothing to suggest that Roche knows (or that it is obvious in the circumstances) that users will assemble “a plurality of fluid delivery devices … wherein the transcutaneous patient access tool is integrated into the exit port assembly”, since (if following the instructions) this would require both devices to be mounted onto (and through) the patient’s skin at once.
338. This point was not pursued with any vigour and, in closing, Roche relied principally on the argument that, as a matter of construction, the claim required a plurality of fluid delivery devices rather than a kit of enough components to enable the user to assemble multiple fluid delivery devices and that no such plurality existed in the Solo kit, nor did Roche intend to provide a plurality of devices.
339. On this claim, on balance I consider that Mr Waugh QC is correct and that the patentee had in mind a situation in which a single remote controller was supplied but plural fluid delivery devices were made available to the user. Given the nature of the claim, I do not consider that the patentee required that a plurality of fluid delivery devices would be used at the same time or that they should be delivered fully assembled. The logic of the patent and the invention is that the patentee claimed a system which involved a non‑disposable element (the remote controller) plus a disposable element (the fluid delivery device). Given the disposable nature of the fluid delivery device, the purpose of Claim 3 is to ensure that the provision of a kit containing multiple fluid devices (or the means to assemble multiple fluid delivery devices) was not arguably outside the scope of the principal claims (Claim 1 ‘a device’; Claim 2 ‘A system including a fluid delivery device’) (emphasis added).
340. I consider that the skilled person would have read the claim in the way contended for by Insulet in all the circumstances of the other claims and the description. On a normal interpretation, the supply of a kit containing the means to assemble more than one fluid delivery device infringes Claim 3 and it is obvious in the circumstances that users would assemble the devices so supplied and use them with the remote controller. The consequence is that there would have been infringement of Claim 3 had Integers 3G and 3H been present in the Solo device which, given the findings on Claim 1, they are not.
Amended Claim 43
341. This claim depends on amended Claims 1, 2 or 3. The claim is to a device, system or kit in which the fluid delivery device receives flow instructions from the separate remote control device, and the housing of the fluid delivery device does not have any user output components.
342. There is a dispute about whether the housing of the Solo device includes “user output components for providing the flow information from the local processor to a user”. Roche relies on a speaker to provide audible feedback to user commands (essentially to confirm successful receipt of quick bolus button presses). Given the construction of the claim language above, I conclude that the Roche device does not infringe.
Amended Claim 45
343. This claim (originally Claim 44 as granted) is to a system comprising (a) a fluid delivery device according to Claim 1 for attachment to a skin surface of a patient, and (b) a remote control device separate to the fluid delivery device with user input and output components.
344. This claim was construed to cover a fluid delivery device which is capable of attachment to the skin of a patient.
345. Neither Roche’s skeleton argument nor its written closing submissions dealt with the infringement of this claim although it was dealt with in Mr Treneman’s evidence. Mr Treneman raised a point which depended on the construction of ‘housing’ and which cannot succeed in the light of the way in which Integer 1G is to be construed. His second point was that it is the pump holder (rather than the micropump assembly) that is adapted for attachment to the patient’s skin and that the device therefore would not fall within the Claims as understood by the skilled person.
346. In response to Mr Treneman’s evidence, Insulet submits that the relevant time to assess whether the assembled device falls within the claim is when the device is assembled and ready to deliver insulin, at which point the micropump and pump holder will be connected and attached to the surface of the skin of the user. Insulet points out in addition that the pump holder is supplied with pre-applied adhesive suitable for being adhered to the patient’s skin.
347. In view of the broad language used in Claim 45, and the way in which it is to be construed, I prefer the evidence of Mr Causey and the submissions of Insulet on this issue and conclude that the Roche device is for attachment to the skin surface of a patient within the scope of Claim 45. The consequence is that there would have been infringement of Claim 45 if Claim 1 had been infringed.
Validity - Introduction
348. A granted patent is presumed to be valid until the contrary is shown. The burden of proof rests with the party disputing validity. Lack of novelty and lack of inventive step are two of the principal bases on which the validity of a patent can be attacked. Roche relies on both of these as against ‘764. There is also an added matter attack.
Novelty/anticipation - the main legal principles
349. Section 2(1) of the Patents Act 1977 provides: “An invention shall be taken to be new if it does not form part of the state of the art”.
350. The principles to be applied when addressing lack of novelty were set out by Lord Hoffmann in Synthon BV v SmithKline Beecham Plc (No.2) [2005] UKHL 59 at [19]‑[33]. There are two requirements: prior disclosure and enablement.
351. There must be a clear and unambiguous disclosure of something which, when performed, necessarily infringes the patented invention. If a document, the disclosure must be construed as it would have been understood by the skilled person at the date of disclosure. Hindsight, informed by the subsequent patent or other developments is to be avoided: the meaning of a document does not change over time. ‘Cherry - picking’ from different aspects within the disclosure of a single document risks straying beyond what would have been disclosed to the skilled person.
352. Enablement means that the skilled person would have been able to perform that which was disclosed. In the present case, an order made at the PTR means that Insulet may not challenge the enablement aspect of the prior art.
353. In Synthon, Lord Hoffmann approved the summary by Sachs LJ in General Tire & Rubber Co v Firestone Tyre & Rubber Co Ltd [1972] RPC 457, 485:
“To determine whether a patentee’s claim has been anticipated by an earlier publication it is necessary to compare the earlier publication with the patentee’s claim. The earlier publication must, for this purpose, be interpreted as at the date of its publication, having regard to the surrounding circumstances which then existed, and without regard to subsequent events.
If the prior inventor’s publication contains a clear description of, or clear instructions to do or make, something that would infringe the patentee’s claim if carried out after the grant of the patentee’s patent, the patentee’s claim will have been shown to lack the necessary novelty, that is to say, it will have been anticipated …
If, on the other hand, the prior publication contains a direction which is capable of being carried out in a manner which would infringe the patentee’s claim, but would be at least as likely to be carried out in a way which would not do so, the patentee’s claim will not have been anticipated, although it may fail on the ground of obviousness. To anticipate the patentee’s claim the prior publication must contain clear and unmistakable directions to do what the patentee claims to have invented ... A signpost, however clear, upon the road to the patentee’s invention will not suffice. The prior inventor must be clearly shown to have planted his flag at the precise destination before the patentee.”
354. Having approved that statement, Lord Hoffmann went on to state at [22]:
“But patent infringement does not require that one should be aware that one is infringing: “whether or not a person is working [an] . . .invention is an objective fact independent of what he knows or thinks about what he is doing”: Merrell Dow Pharmaceuticals Inc v H N Norton & Co Ltd [1996] RPC 76, 90. It follows that, whether or not it would be apparent to anyone at the time, whenever subject-matter described in the prior disclosure is capable of being performed and is such that, if performed, it must result in the patent being infringed, the disclosure condition is satisfied. The flag has been planted, even though the author or maker of the prior art was not aware that he was doing so.”
355. Lord Hoffmann explained the difference between anticipation and obviousness at [25]:
“… it is this requirement that performance of an invention disclosed in the prior art must necessarily infringe the patent which distinguishes novelty from obviousness. If performance of an invention disclosed by the prior art would not infringe the patent but the prior art would make it obvious to a skilled person how he might make adaptations which resulted in an infringing invention, then the patent may be invalid for lack of an inventive step but not for lack of novelty.”
356. He then explained the difference between disclosure and enablement at [30]:
“… I have explained that for the purpose of disclosure, the prior art must disclose an invention which, if performed, would necessarily infringe the patent. It is not enough to say that, given the prior art, the person skilled in the art would, without undue burden, be able to come up with an invention which infringed the patent. But once the very subject-matter of the invention has been disclosed by the prior art and the question is whether it was enabled, the person skilled in the art is assumed to be willing to make trial and error experiments to get it to work.”
357. Finally, at [32] Lord Hoffmann turned to the role of the person skilled in the art when considering disclosure:
“In the case of disclosure, when the matter relied upon as prior art consists (as in this case) of a written description, the skilled person is taken to be trying to understand what the author of the description meant. His common general knowledge forms the background to an exercise in construction of the kind recently discussed by this House in Kirin-Amgen Inc v Hoechst Marion Roussel Ltd [2005] RPC 9. And of course the patent itself must be construed on similar principles. But once the meanings of the prior disclosure and the patent have been determined, the disclosure is either of an invention which, if performed, would infringe the patent, or it is not. The person skilled in the art has no further part to play.”
Obviousness/inventive step - the main legal principles
358. Section 3 of the Patents Act 1977 provides: “An invention shall be taken to involve an inventive step if it is not obvious to a person skilled in the art, having regard to any matter which forms part of the state of the art…”
359. It is for Roche to establish on the balance of probabilities that the skilled person or team would have considered it obvious to move from the teaching of the prior art to the invention claimed in the Patent.
360. The Supreme Court addressed obviousness in Actavis Group PTC EHF v ICOS Corp [2019] UKSC 15 at [60]:
“In addressing the statutory question of obviousness in section 3 of the 1977 Act it is common for English courts to adopt the so-called Windsurfing/Pozzoli structure which asks these questions:
“(1)(a) Identify the notional ‘person skilled in the art’;
(b) Identify the relevant common general knowledge of that person;
(2) Identify the inventive concept of the claim in question or if that cannot readily be done, construe it;
(3) Identify what, if any, differences exist between the matter cited as forming part of the ‘state of the art’ and the inventive concept of the claim or the claim as construed;
(4) Viewed without any knowledge of the alleged invention as claimed, do those differences constitute steps which would have been obvious to the person skilled in the art or do they require any degree of invention?”
(Pozzoli SPA v BDMO SA [2007] EWCA Civ 588; [2007] FSR 37, para 23 per Jacob LJ). The fourth question is the statutory question and the first three questions or tasks, the second and third of which involve knowledge and consideration of the invention, are a means of disciplining the court’s approach to that fourth question …”
361. The task is to approach the prior art with care as the skilled person would have done at the date of the prior art, in the light of the CGK and to “… evaluate all the relevant circumstances in order to answer a single and relatively simple question of fact.” Medimmune Ltd v Novartis Pharmaceuticals UK Ltd & Ors [2012] EWCA Civ 1234 at [93], per Kitchin LJ.
362. The Court of Appeal explained in Molnlycke AB v Procter & Gamble Ltd [1994] RPC 49 (CA) at page 112, line 40 that the Court will be assisted by the evidence of the expert witnesses as to whether or not the relevant step would have been obvious to the skilled person in the light of the state of the art. Other evidence may also be relevant, but the evidence of the experts takes the primary role and must be carefully assessed to identify what the notional skilled person would have regarded as obvious.
363. As summarised by Jacob J in Unilever PLC v Chefaro Proprietaries Ltd [1994] RPC 16, at page 580:
“… the test is that set out in the statute and none other. Any other verbal formula leads to danger. In operating the test, the Windsurfing logical structure is helpful. The question is one of overall fact. Inferences from secondary evidence are relevant.”
364. The CGK was discussed at paragraphs 29-105.
365. Each of the claims in respect of which inventive step is in issue has been construed above and that construction is referred to as necessary below.
366. The identity of the skilled person has been discussed above at paragraphs 24-28. However for the purposes of the analysis below it is worth saying a few more words about the attributes of the skilled person in the context of obviousness.
367. Prior art will be read with the prejudices, preferences and attitudes that the skilled person had at the priority date as explained in Asahi Medical Co Ltd v Macropharma (UK) Limited [2002] EWCA Civ 466 at [21] per Aldous LJ. Although the skilled person is assumed to be interested in the relevant field of technology there is no assumption of knowledge before reading the patent that any particular piece of prior art solves the problem under consideration. It is therefore possible that, having read the prior art, the skilled person would not have found the document useful or worth further development as stated by Laddie J in Inhale Therapeutic Systems Inc v Quadrant Healthcare Plc [2002] RPC 21 at [47].
368. The skilled person need not be someone in an established business in the field. A patent is obvious if it is obvious to a skilled person who is working for a new entrant:
“Obviousness is tested against the mental and developmental norm of a notional uninventive person skilled in the art. In doing that the law is protecting not only established businesses which may wish to adopt new products, processes or designs or modify existing ones but also the new entrant who has employed persons skilled in the art to help him get into the market. Each of those categories of trader must be free to adopt what is obvious. Therefore it is legitimate to approach the prior art as if it had been collected and put on the desk of the new entrant at the priority date.” Brugger v Medicaid Ltd (No. 2) [1996] RPC 635 at page 653, per Laddie J.
369. In this case it is important, as already noted above, to recall that the skilled person is a notional construct who will not have all of the attributes of either expert, and that the particular backgrounds of the experts is relevant context when assessing their evidence as to obviousness.
The prior art
PhiScience - published 25 May 2000
370. PhiScience is relied on to support both a novelty attack against Claim 2 of the Patent and an obviousness attack against all the Claims in Issue. It is argued by Roche that PhiScience anticipates the main invention disclosed by Integer 1H of the Patent and that all other claims are either clearly disclosed or obvious.
371. PhiScience is an international patent application filed in German. An English translation in agreed form was supplied. It was published on 25 May 2000 and is entitled “Portable device and method for the mobile supply of medicaments with wireless transmission of data for control or programming purposes”. It starts by explaining that “The invention relates to a portable device as well as a method for supplying medicaments via a mobile, non-implantable means for basal rates or basal profiles and/or bolus doses with wireless operation or programming.”
372. The invention is summarised as:
“… based on the problem of providing a device that is unobtrusive with regard to wearer comfort and operation, and which also requires no surgical intervention, with the infection and anaesthesia risk associated with the same. One solution to this problem according to the invention is stated in patent claim 1. Further developments of the invention are the subject of claim 2. The device according to the invention preferably consists of two components: dispensing unit (non-implantable) and operating unit.”
373. The main focus of the application is on the “operating unit” which is responsible for control and programming of the device and for displaying data. This is essentially a remote control device. The application identifies prior art documents which it says do not describe a remote control or external operating unit, followed by various prior art documents which it says have wireless control but relate to implantable devices. It explains that “The aspect of a simple, unobtrusive operation of the external control unit has not been taken into consideration to date.”
374. The “Illustration of the invention” starts at page 4. At the foot of the page, it states:
“With generally commercially available insulin pumps the operating and control elements require most of the space in addition to the medicament reservoir (for example ampoule) and the drive (for example motor). The externalisation of an operating unit (apart from minimal emergency operating elements such as an emergency off and an emergency bolus button) to a location that is spatially separate from the rest of the dispensing unit therefore means a substantial space saving with regard to the dispensing unit and allows a smaller, and therefore more inconspicuous construction. The regular operation of the pump should not necessarily be on the pump itself, nor should it be necessary for the operating unit that the dispensing unit is provided in a conspicuous way or even needs to be physically (for example via a cable) connected with the operating unit.”
375. At the bottom of the following page it is explained that: “The dispensing unit and operating unit therefore both have transmission as well as receiving means and communicate wirelessly with one another.” The Application goes on to explain that the dispensing unit also includes a reservoir, an energy supply, the drive, the electronic control and “possible further construction elements (for example the said emergency operating guarantee rudimentary basic functions…)”.
376. The operating unit is said to be “housed in an inconspicuous object of daily life, which can also fulfil other - suitable - functions (for example, a watch, pocket calculator, electronic pocket databank, “hand-held computer”, “palm-top computer” or similar miniature computers, key rings, pocket cosmetics set, wallet, etc.)”. It “allows the input of data as well as the operation and programming of the dispensing unit”; “transmits all necessary data for a quantitative and timely control of the medicament dispensation to the dispensing unit”; and “controls the dispensing unit on the basis of data regularly exchanged by means of the communication means”.
377. On page 7 the Application states:
“In one further development of the embodiment the dispensing unit is directly connected with the body of a patient by means of a cannula, or indirectly by means of a catheter. To transport liquid medicaments from an ampoule the torque of a motor is transmitted to a rotatable spindle with a suitable drive, which preferably has a worm gear drive. The rotation of the spindle moves a cartridge in the direction of the piston of the ampoule, such that the piston extracts liquid from the ampoule.”
MiniMed - published 2 March 2000
378. MiniMed is relied on to support an obviousness attack. The fundamental position taken by Roche is that it would be obvious in the light of Minimed: (i) to move all controls and display from the infusion device to the remote control; and (ii) then to locate the pump at the infusion site, directly connecting it to a cannula and thereby solving the drawbacks associated with the use of external tubing. This would result in a device having the characteristics comprising the main inventive concept of the Patent and it is submitted that all other claims of the Patent are either clearly disclosed or obvious.
379. MiniMed is an international patent application entitled “External infusion device with remote programming, bolus estimator and/or vibration alarm capabilities”. At a high level, it consists of an ambulatory device, connected to the patient via tubing and an infusion set, and a remote control. The invention is described as relating to external infusion devices and, “in particular embodiments, to a medication infusion device that includes the capability to be remotely controlled, a bolus estimator to determine the dosage to be administered by the infusion device, and a vibration alarm”.
380. The system is not limited to insulin. However, the section which give the background to the invention discusses the way in which insulin is provided to those with diabetes and a CSII insulin pump is given as the preferred embodiment as shown below.
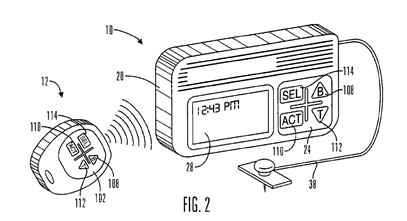
381. After a general introduction to insulin pump therapy, MiniMed notes at page 2 that:
“One drawback is the inability to conceal an external infusion pump and catheter tubing from view. Many users desire to hide the external pump under clothing so as not to seem different from normal people. However, this is inconvenient or impractical, especially for diseases such as diabetes, since a user must have ready access to the external pump for monitoring or administering extra amounts of medicament (i.e., boluses during the course of the day). If a user has concealed the external pump, the user must partially undress or carefully maneuver the external pump to a location that permits access to the display and keypad.”
382. MiniMed discloses an improved external infusion device that overcomes certain limitations of existing devices identified as:
· the inability to conceal the pump and tubing from view (e.g. under clothing) while having ready access to the device in order to program it;
· limiting access to pump operational parameters, where it may be desirable to restrict options accessible, e.g. to children or the elderly;
· calculating the amount of bolus insulin to deliver after meals, which involves difficult calculations using formulas and guesswork; and
· the need to prime external infusion pump to remove gas bubbles in the reservoir and tubing (e.g. by shaking the reservoir and expelling fluid), which wastes a quantity of medicine.
383. The key elements of MiniMed are:
· an external infusion device with a receiver for receiving commands from a “remote commander” allowing it to be operated when concealed;
· the remote commander (also referred to as the “RF programmer”) allowing the user to perform basic programming steps without using the keyboard or LCD screen on the pump, and transmit those commands to the infusion device;
· an infusion system comprising (i) an external infusion device having a housing, receiver, processor and “indication device” (for confirming user input); and (ii) a “remote commander” for generating commands and sending them to the device.
384. A schematic of the MiniMed device is below. A key aspect is highlighted.
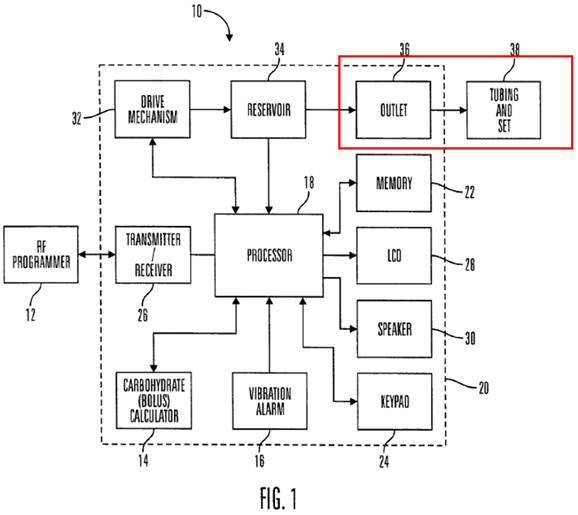
PhiScience - Novelty
385. Roche’s case on anticipation rests on PhiScience. It is limited to Claim 2. The integers in respect of which there is a dispute are:
· Integer 2C/1B: an exit port assembly adapted to connect to a transcutaneous patient access tool (the dispute relating to disclosure of an EPA also goes to Integers 2D, 2F and 2G/1C, 1D and 1F);
· Integer 2A/1G: wherein the housing is free of user input components for providing flow instructions to the local processor;
· Integer 2I/1H: wherein the transcutaneous patient access tool is integrated into the exit port assembly;
· Integer 2M: and further comprising a proximity alarm
General points on disclosure
386. There are one or two general points which it is convenient to deal with at this juncture.
387. The first is whether PhiScience disclosed only that the remote control or operating device should be part of another electronic device. This argument was advanced by Insulet as a reason not only why PhiScience does not anticipate the patent but also why it cannot sensibly support an argument on inventive step.
388. Insulet argues that, while the skilled team would be interested in the idea that two separate components (a dispensing unit and a remote control) should be used to deliver insulin, PhiScience would not be seen as a practical starting point because the suggestion that the remote control should be combined with a multifunction electronic device would have been seen as impractical and dangerous.
389. This argument was supported by the written evidence of Mr Causey who expressed the view that there are significant issues with that type of dual-purpose electronic operating unit, particularly relating to reliability and safety. He noted that PhiScience provides no explanations as to how to overcome those challenges, so the skilled person would have regarded the idea as “impractical and potentially dangerous” and not one that he would have pursued. Later in his report, he identifies this as a “key difference between the teaching of PhiScience and the inventive concept of [the Patent]”.
390. Leaving aside the questions as to the practical challenges which the skilled person might face, which are dealt with as far as relevant below, it is not open to Insulet to make the general point about electronic devices as the relevant passage clearly also discloses non‑electronic objects which can house the remote control, including an analogue watch, a key ring, a pocket cosmetics set and a wallet. During his cross‑examination, Mr Causey accepted that if there were practicality or safety concerns with the use of dual/multi-function electronic devices, then the use of non-electronic devices was also disclosed.
391. The second general issue was the weight to be attributed to PhiScience. Mr Waugh QC noted that PhiScience had been contrasted with the invention of the Patent at paragraph [0010] which he submitted made it unpromising as a basis to attack the patent. As a point of practicality this may have some weight, but it cannot undermine the value of a disclosure that meets the tests set out in Synthon (in this case, clear and unambiguous disclosure only, as enablement is not in issue).
392. Mr Waugh QC also referred to PhiScience as a ‘paper patent’, noting that the invention had never been commercialised, that the application had been withdrawn before grant, and that the inventors were not well known in the Insulin delivery field. He also submitted that the teaching of PhiScience was high level and lacked detail, referring to Mr Causey’s evidence that PhiScience was a collection of “many different ideas without clear practical detail of how those ideas should be combined and implemented”. Mr Waugh QC suggested that, as PhiScience was teaching a general purpose device rather than one specifically directed towards insulin, this could limit the understanding that the skilled addressee would take from the patent (although he did accept that PhiScience disclosed its principal embodiments as directed towards the delivery of insulin via a CSII pump). Given all of those general considerations, Mr Waugh QC suggested that PhiScience should be approached with significant caution.
393. The context in which any patent is to be construed is relevant to its construction and to how the skilled addressee would understand the language used. It is true that “Patent law has for a long time, and rightly, regarded with particular suspicion arguments based on a suggestion that long disregarded unused proposals render later inventions obvious” (Grimme at [59] per Jacob LJ), but this is part of the overall context rather than a suggestion that inventions which have not been commercialised fall into a special category. Ultimately, what matters is the teaching which can be derived from the document when properly construed, having regard to the context.
Integer 2C/1B: an exit port assembly adapted to connect to a transcutaneous patient access tool
Submissions/evidence
394. PhiScience states that “the dispensing unit is directly connected with the body of the patient by means of a cannula, or indirectly by means of a catheter.”
395. Roche contends (with support from Mr Treneman) that the statement that the dispensing unit can be connected to the patient’s body “directly by means of a cannula’ means that no connecting tubing between the dispensing unit and the cannula would be required; whilst a ‘conventional tube and set’ could also be used which would then be “indirectly by means of a catheter”. Roche contends that on its clear meaning the distinction in PhiScience is simply between a direct/tubeless connection between the infusion device and the body using a cannula; and an indirect connection between the infusion device and the body involving a standard infusion set of catheter/tubing and a cannula.
396. Roche further submits that if this is correct there would have to be something connecting the fluid path inside the dispensing device to the fluid path through the cannula, which would be an EPA and that there would also be a TPAT to which the EPA would be adapted, as the cannula is disclosed as being directly attached to the fluid delivery device.
397. Insulet submits that Roche’s contention is clearly wrong, relying primarily on the evidence of Mr Causey. Insulet also criticises Mr Treneman’s approach.
398. Mr Causey’s original reading of this disclosure suggested that PhiScience distinguishes between: (a) the use of a standard tubing and cannula (directly by means of a cannula); and (b) a system used in a hospital setting with larger tubing (indirectly by means of a catheter). His first report noted that infusion sets with cannulas connected to the pump by tubing were the standard approach to the provision of subcutaneous infusion for insulin and inferred that “The inventors therefore seem to have been seeking to cover both patient-administered, home-based treatment and more specialist treatments administered by healthcare professional in hospital environments.” Having reached this conclusion rather tentatively in his first report, his views had become firmer by the time of his second report, where he explained:
“At paragraph 154 Mr Treneman states that the second paragraph on page 7 of PhiScience describes a device with no tubing between the device and the TPAT. As I discussed at paragraph 80 of my First Report, I would read this sentence to be distinguishing between a traditional tubing and infusion set with a cannula and a catheter (typically larger and used in hospitals). Given the references to general commercially available insulin pumps, the lack of references to body mounting, the lack of commentary on redesigning the pump to allow a cannula to connect to the device and the lack of any suggestion that this is a significant modification I do not think this sentence in PhiScience is describing or suggesting a cannula which is integrated into the device without tubing. Had anything as radical as Mr Treneman suggests have been contemplated I would expect it to have been set out more clearly and explained in some detail.”
399. During cross‑examination, Mr Causey agreed that the disclosure of PhiScience relates to pumps used at home by individuals. He then explained that in fact, rather than relating to hospital tubing, as originally suggested, the distinction between “directly by means of a cannula” and “indirectly by means of a catheter” was based on a distinction between a standard tubing and cannula (directly by means of a cannula) and the “non-standard Disetronic DiaPort” (indirectly by means of a catheter). He explained that the Disetronic DiaPort was “a surgically implemented device that the H-Tron pump was connected to” which was also a catheter. This means of connection between the dispensing unit and the patient had not been mentioned in his written evidence.
400. Mr Causey remained clear during his cross-examination that when PhiScience said “directly by means of a cannula” it would have been taken by those in the field at the time to mean a standard tube and set, terminating in a cannula as those were the commercially available products at the time. His evidence was that any other understanding of such a limited statement would have been unthinkable. He explained his view that at the time:
“There was no way to get rid of the tubing because there was no configuration of a pump and one imagined, or at least that we imagined where you could get rid of the tubing because that would have required the device to be body-adhered and we knew technology could not be body-adhered. It was simply too large. There was no way to imagine removing the tubing.”
401. When pressed by Mr Tappin QC, Mr Causey clarified that he was speaking from the perspective of MiniMed. However, in the context of the discussion of CGK above, I accepted that MiniMed and Disetronic formed the bulk of the industry at the time and that for a number of years before the priority date all of those who were manufacturing and designing pumps had done so using a tubed configuration and with incremental improvements (see paragraphs 63-66 above) so that the skilled person would have considered the use of external tubing as the accepted approach at the priority date. This gave some support to Mr Causey’s view as a starting point on the potential meaning to a skilled person of the first suggested configuration “directly by means of a catheter”.
402. Nevertheless, the weight to be attributed to this evidence would be significantly reduced if there were no plausible alternative meaning for PhiScience’s second suggested configuration “indirectly by means of a catheter”. As a matter of construction, it must be assumed that the inventor meant different things by the two alternatives so if the first configuration addressed tubing and a cannula, the second must address something else.
403. Having accepted that his first suggestion of tubing used in a hospital setting would not have been what PhiScience would have had in mind when disclosing connection with the dispensing unit “indirectly by means of a catheter”, Mr Causey concluded that, given his view of the mindset of those in the field at the time, PhiScience would have been understood to mean something that did not terminate in a cannula, and therefore distinguishing between a standard tubing and set and an alternative solution whereby “insulin is being infused by a tubing, in the case of the application revealed in late 1999, through a DiaPort which is indirect into the body. It is an opening through the skin”.
404. Mr Tappin QC criticised this evidence on the grounds that, in summary:
· it was not CGK;
· it was a late arrival in the proceedings, having first emerged in Mr Treneman’s cross-examination bundle;
· PhiScience could not have had the DiaPort in mind as all of the priority dates for PhiScience pre-dated the earliest mention of the DiaPort (at an American Diabetes Association Meeting in June 1999) during which it was only briefly mentioned as being experimental and unavailable; and, finally
· the insertion of the DiaPort required surgery, while PhiScience is not concerned with systems that require surgical intervention.
405. The final point was mentioned by Mr Treneman during cross-examination as a reason why the skilled person would be much more likely to read the language as referring to a standard infusion set rather than something requiring surgical intervention.
406. On balance, I do not consider that Mr Causey’s evidence about the DiaPort is persuasive primarily as: (a) it was not the construction that struck him as plausible in either his first or his second written report; and (b) that the DiaPort required surgical intervention, contrary to the teaching of PhiScience: “The invention is based on the problem of providing a device that is unobtrusive with regard to wearer comfort and operation, and which also requires no surgical intervention, with the infection and anaesthesia risk associated with the same.”
407. In the light of that conclusion, Mr Causey’s evidence about the meaning that the skilled person would attribute to the first configuration is less persuasive. It rests on Mr Causey’s strong conviction that the mindset of the skilled person was such that the concept of a connection which did not involve tubing would not be the most likely meaning for any form of connection between the dispensing unit and the body of the patient. However, in circumstances where no meaning for the second configuration has been suggested, the context suggests that the skilled person would have considered further what PhiScience had in mind by the words that had been chosen.
408. Having already discussed the criticism of Mr Causey’s evidence by Roche, I should make clear that Mr Treneman’s evidence was equally heavily criticised by Insulet, both as to his view of the meaning of “directly by means of a cannula” and as to his conclusion that this inevitably led to the identification of an exit port assembly adapted to connect to a transcutaneous patient access tool.
409. The first main criticism is that in his first report Mr Treneman recasts the disclosure of PhiScience to read it as teaching that “delivery of insulin to the patient is by cannula directly connected to the dispensing unit” (emphasis added by Insulet) whereas the language of PhiScience is in fact that “the dispensing unit is directly connected with the body of the patient by means of a cannula, or indirectly by means of a catheter.” Insulet submits that this reframing of the language leads Mr Treneman to proceed from the wrong starting point and that he is therefore wrong when he considers that PhiScience discloses a tubeless pump because this is not how the skilled person would have understood the passage, given the universal use of external tubing at the time. Insulet submits that this demonstrates that Mr Treneman’s reading of PhiScience suffers from hindsight, and it is only this which leads him to infer that the word ‘directly’ refers to the absence of external tubing, rather than the conventional use of an infusion set.
410. The second main criticism is that Mr Treneman has taken the statement out of context and that nothing else in PhiScience suggests any change to the existing approach. Mr Waugh QC submitted that Mr Treneman’s reading was incredible on the basis that PhiScience does not claim a “whole new generation of tubeless patch pump”; its authors do not seem to consider that this is covered by the language used; the entire focus of PhiScience is on the remote device; and the application was abandoned before grant.
411. In closing, Mr Waugh QC submitted that to deduce the absence of tubing Mr Treneman must rely on inference and deduction, as PhiScience does not, in terms, refer to doing away with external tubing. Mr Waugh QC stressed that Mr Treneman has accepted that PhiScience does not say anything about tubeless design.
412. Finally, Insulet submits that there is no disclosure at all of an EPA.
413. I do not consider the evidence of Mr Treneman to be flawed in the ways in which Insulet suggests. Overall, I found his views as to the meaning of this aspect of PhiScience to be more helpful than those of Mr Causey although it is, of course, necessary to look at those words in context and with the purpose of the inventors in mind. I do not accept that he re-framed the language in a way that led him into error. He repeated the specific language used by PhiScience in his written evidence but reiterated that it should be interpreted in the way that he suggested. During his cross-examination, he clearly explained his reasons for reading the language in the way that he had, again reciting the precise language used by the authors of PhiScience, and explaining what he understood it to mean, notwithstanding some technical difficulties during this portion of his cross‑examination, and some rather lively exchanges with Mr Waugh QC.
414. Of course, the suggestions of hindsight and undue literalism must be borne in mind. Mr Treneman’s role is to do the best he can to put himself in the shoes of the skilled person at the date of PhiScience, so as to assist the Court in construing that document. In construing the PhiScience disclosure, I shall bear in mind the suggestion of hindsight when considering the overall weight to be attributed to Mr Treneman’s evidence. However, it is equally important to bear in mind that Mr Causey’s reading of the language and his strong rejection of a possible construction that did not involve tubing was affected by his personal experience at MiniMed so that he found it difficult to approach the topic without a degree of commitment to the tubed concept that might not have been so strongly shared by the notional skilled person (who need not necessarily have been working specifically in the field of insulin pumps).
415. In the light of the views of both experts, which I found helpful, I must now consider what, on balance, the skilled person would have understood the author of the description to have meant, bearing in mind his common general knowledge, starting with the language used by the inventor in the context of the patent and the inventor’s purpose.
416. The language used clearly envisages two alternative configurations. That being so, any construction must involve the identification of two alternative options which fit within the overall context and purpose of the patent.
417. As discussed in the context of CGK, the word ‘catheter’ is not a term of art and the skilled person would not have had a settled view on the meaning of catheter in the context of infusion pumps at the priority date. In particular, it has not been established that the tubing and cannula set together would be commonly referred to as a catheter or that the word cannula incorporated the use of tubing.
418. Looking at the overall teaching of PhiScience, the focus of the inventors is on the remote control, rather than on the dispensing unit, to which they pay relatively little attention, although it is specifically mentioned in Claims 1 and 2 (as well as a further mention in Claim 17). The disclosure on page 7 is the only mention of how the dispensing unit is to be connected to the patient. It make no reference to tubes. This disclosure occurs in a context where the principal focus is the externalisation of controls to the separate remote control unit. The disclosure on page 7 is the only discussion of how the device is to be carried or worn. It is clear from the description that PhiScience was focussing on minimising the size of the dispensing unit by reducing the space required for the operating elements so as to achieve “the smallest possible and unobtrusive construction of the pump together with unobtrusive operation” (see PhiScience, page 3).
419. The concept of an unobtrusive device which is comfortable to wear was clearly important to the inventors, as they mention the problem of providing such a device in the first paragraph describing the ‘illustration of the invention’ and the concept is repeated consistently throughout the description, mentioning the small size and unobtrusive nature of the dispensing unit. It is in this context that the authors of PhiScience discuss the attributes of the dispensing unit and give two alternatives for the connection of the unit “directly with the body of a patient by means of a cannula, or indirectly by means of a catheter”.
420. Taking the evidence in the round and weighing it up, I conclude that, when the inventors in PhiScience drew a distinction between the dispensing unit being “directly connected with the body of the patient by means of a cannula, or indirectly by means of a catheter”, the most likely reading was that they meant to distinguish between a conventional tubing and set, as required for a device that could not be directly connected to the body of a patient owing to its size and weight, and one which could be so connected because the operating controls had been removed meaning that it could be small and unobtrusive and capable of direct connection to the body without tubing.
421. When this potential meaning was put forward by Mr Treneman, Mr Waugh QC objected that it could not be correct as none of the claims of PhiScience go to a ‘tubeless’ pump and that it would be very strange for the inventors not to have included in their patent a specific claim related to such a new class of device or not to have commercialised such a device. Mr Waugh QC went so far as to say:
“Indeed, it is somewhat incredible to suggest, as Mr Treneman does, that PhiScience is teaching a whole new generation of tubeless patch pump, yet (i) its inventors have not realised that, (ii) its 23 detailed claims do not claim that, (iii) its focus is not on the dispensing device or infusion site at all, but on the remote device, and (iv) the patent application was abandoned before grant and never commercialised.”
422. I disagree. If the language in the specification is sufficiently clear in context, it would be wrong to interpret it in a way that takes account of some perceived commercial failing on the part of the inventor - or any subsequent conduct or lack of conduct on the part of the inventor. To seek to narrow the disclosure in the specification by reference to the claims (or lack of them) would also be wrong. It is clear that the claims and the specification perform different functions, as described by Laddie J in Merck & Co Inc v Generics (UK) Ltd [2003] EWHC 2842 (Pat) at [38]:
“The purpose of a patent is to convey to the public what the patentee considers to be his invention and what monopoly he has chosen to obtain. These are not necessarily the same. The former is primarily to be found in the specification and the latter is primarily to be found in the claims. Although he is not deemed to be a patent lawyer, the patentee should be taken to be aware of the primary and rather different purposes of the specification and the claims when drafting his patent. So, the patentee must be taken to know the framework of form and purpose when he drafts his patent. It is his duty to communicate his invention and his assertion of monopoly to the public in language it will understand. He is warned by the Protocol that his exclusive rights will not necessarily extend to everything which, from a reading of the specification, it can be seen that he contemplated. Furthermore, the drafting of the specification and claims has to be considered against the background that no one is forced to apply for a patent or to seek as wide protection as possible. The patentee can be taken to be aware of the fact that there is always a balance to be achieved between width of protection and validity. It is up to the patentee to choose the level of risk he wishes to run.”
and it cannot be assumed or inferred that the scope of the claim is (or need be) the same as the matters covered in the description. This was reiterated by Lord Hoffmann in Kirin‑Amgen Inc v Transkaryotic Therapies Inc (No.2) [2005] RPC 9 where he stated at [33]: “There is no presumption about the width of the claims. A patent may, for one reason or another, claim less than it teaches or enables.”
423. It is also established that the knowledge of the inventor is irrelevant to the question of whether something has been disclosed. I shortly discuss what (if anything) is and is not anticipated by PhiScience, but that has nothing to do with the knowledge or understanding of the inventor. This follows from the authorities, including Synthon:
“If the prior inventor’s publication contains a clear description of, or clear instructions to do or make, something that would infringe the patentee’s claim if carried out after the grant of the patentee’s patent, the patentee’s claim will have been shown to lack the necessary novelty, that is to say, it will have been anticipated … It follows that, whether or not it would be apparent to anyone at the time, whenever subject-matter described in the prior disclosure is capable of being performed and is such that, if performed, it must result in the patent being infringed, the disclosure condition is satisfied. The flag has been planted, even though the author or maker of the prior art was not aware that he was doing so.”
424. In conclusion, PhiScience discloses an embodiment of a fluid delivery device which could be directly connected to the body of a patient using a cannula alone as an alternative to the conventional method using an infusion set involving tubing whereby there is no direct connection between the cannula and the device.
425. I turn next to whether the disclosure of a tubeless attachment in PhiScience is a clear and unambiguous disclosure of something which, when performed, necessarily infringes the patented invention meaning that the invention is anticipated.
426. I have concluded that PhiScience disclosed a tubeless connection between the body and the infusion device using a cannula. No issue as to enablement arises. The dispute here therefore turns on whether PhiScience disclosed the specific attributes said to set the Patent apart - the EPA, the TPAT and the integration between them.
427. Insulet has stated that it considers the core of its invention to be a “tubeless patch pump”. While this is not specifically claimed, it is submitted by Insulet that this concept is captured in Integers 1B, 1H and the other integers of the Patent which refer to “an exit port assembly adapted to connect to a transcutaneous patient access tool” as it is these which allow a tubeless connection.
428. Insulet submits that PhiScience does not disclose anything about an EPA or a TPAT and that there is thus no ‘planting of the flag’. I note that Mr Treneman explained that these were new terms used by Insulet in the Patent which he had not previously encountered.
429. Neither term is to be found in PhiScience. This does not mean that the same subject matter is not disclosed. There is no requirement for equivalence of language between the prior art and the invention. All that is required is that the teaching is the same. Where specific language is used by a patentee to describe aspects of his invention that does not necessarily enable him to distinguish it from the prior art. Prior art may disclose an invention even though the author of the prior art document may not be aware of the fact that he is ‘planting his flag’ on a particular territory. This was clearly explained by Lord Hoffmann in Merrell Dow Pharmaceuticals Inc v HN Norton & Co Ltd [1996] RPC 76 at page 88 referring to the “infinite variety of descriptions under which the same thing may be known.”
430. To understand whether the prior art discloses the same teaching as the Patent, it is necessary to consider whether doing what is suggested by PhiScience would necessarily infringe the claims which involve the use of an EPA or a TPAT.
431. To recap on construction of those terms, I concluded that in the Patent:
· EPA is a means of connecting the flow path from the reservoir to the flow path through the TPAT (see paragraphs 145-148 above); and
· a TPAT is an element that pierces the skin and enables continued transcutaneous infusion of insulin such as a needle cannula or array of microneedles (see paragraphs 149-150 above).
432. PhiScience discloses a direct connection between the fluid delivery device and the body of a patient using a cannula. A cannula is a TPAT.
433. The remaining question is whether PhiScience discloses a means of connecting the flow path from the reservoir to the flow path through the cannula. I found it helpful to understand what the experts considered would necessarily need to happen under the teaching of PhiScience to assess the extent to which PhiScience contained an unambiguous description of something that would infringe the patentee’s claim, satisfying the requirement that the subject matter of the invention had been disclosed so that, to the extent necessary, the person skilled in the art would be assumed to be willing to undertake trial and error experiments to make it work.
434. Both experts agreed that the teaching in PhiScience meant that there would need to be something connecting the fluid path inside the device from the reservoir to the fluid path through the cannula/TPAT. As a matter of construction, that is the same as an EPA in the language of the Patent and the teaching is the same.
435. To the extent that some integers of the Patent require an integrated TPAT (Integers 1H, 2I, 3I, 42A, 45B), I conclude that implementing the invention in PhiScience would also necessarily infringe this aspect of the Patent given that those claims are to be construed as requiring integration between the TPAT and the EPA at the time of use and that Insulet’s purpose was to claim a device in which, at the time of use, two elements (described as the EPA and TPAT) combined together to allow fluid to flow between them and directly through the transcutaneous element into the user.
436. If the disclosure in PhiScience were to be performed it would necessarily infringe the aspects of the Patent which teach an EPA adapted to connect to a TPAT and also those which teach an integrated TPAT. Those integers are therefore anticipated.
Integer 2A/1G: wherein the housing is free of user input components for providing flow instructions to the local processor
437. It is common ground that PhiScience discloses and prefers a device in which the dispensing unit has emergency buttons:
“In a preferred variation of the above embodiments the dispensing unit has emergency operating elements (such as for example an emergency off and an emergency bolus button).”
438. Both experts also agreed that PhiScience discloses as one possibility that the dispensing unit could have no operating controls, with all the controls instead being on the remote control. In other words, it would be free of user input components for providing flow instructions to the local processor.
439. Mr Tappin QC submitted that disclosing a feature as optional also discloses the product without that feature, relying on Gillette v Anglo-American (1913) 30 RPC 465 per Lord Moulton at page 481 lines 18-32 and that therefore the skilled person would have understood PhiScience to be disclosing a device without buttons.
440. Mr Waugh QC did not reply specifically to these submissions but concentrated on pointing out inconsistencies between Roche’s position on this aspect of PhiScience and its position on infringement of Integer 1G.
441. I conclude that Integer 1G of the Patent is anticipated by PhiScience in the light of the teaching of the optional feature.
442. One issue raised by Mr Tappin QC was whether the scope of a patent for validity must be the same as its scope for infringement. He noted that the issue might be live in this case in the event that the Patent extended to a device with some electromechanical controls on the housing and that PhiScience did not disclose a system in which all the controls are removed from the fluid delivery device. He referred to the recent authorities in which the issue has been discussed, noting that both Floyd LJ in IceScape (at [98]) and Terrell (19th edition, at 9-48 - 9-55) treat the issue as unresolved. In the light of the evidence, the question is moot and I need not deal with it further.
Integer 2I/1H: wherein the transcutaneous patient access tool is integrated into the exit port assembly
443. I have dealt with Integer 2I/1H above and have concluded that it is disclosed.
Integer 2M: and further comprising a proximity alarm
444. Roche contends that PhiScience discloses a proximity alarm, citing page 10:
“In a further preferred variation of the above embodiments the dispensing unit and/or the operating unit have alarm transmitters…which trigger an alarm in critical situations (for example an extended period where there is no connection between the dispensing unit and the operating unit).”
445. Insulet contends that this is not an unambiguous disclosure of a proximity alarm.
446. Mr Treneman explained that this would be understood by the skilled person as functioning as a proximity alarm because it would be triggered when there was no connection between the pump and the remote control for an extended period because the devices were too far apart.
447. Mr Causey disagreed, saying that this was not a proximity alarm because it is triggered by time rather than distance.
448. Mr Waugh QC submits that Roche’s argument on this integer must fail because an alarm for connection failure is a distinct idea which does not carry with it a clear and unambiguous teaching to produce a proximity alarm. Connection failure could occur for any number of reasons unrelated to proximity; thus a connection failure alarm may sound in different circumstances to a proximity alarm.
449. Mr Tappin QC disagreed with this view. During cross‑examination of Mr Causey, Mr Tappin QC explored the disclosure of PhiScience. Mr Causey’s position was that the inventive step in the Patent which rendered it novel, and extended to something not disclosed by Phi Science, was the specific question of range and how that is defined as a function of signal strength. Mr Tappin QC pressed him on the distinction between Mr Causey’s description of ‘range’ as a triggering aspect for the alarm and PhiScience’s disclosure of an alarm when there is a critical situation, including an extended period without connection. Mr Causey’s view was that the key difference was the setting of the power level to be a subset of the maximum range.
450. On this issue I prefer the evidence of Mr Treneman. I consider that the skilled person reviewing PhiScience in context, given that a remote control is being taught and the safety critical nature of the system, would have considered excessive distance between the remote control and the device to be a critical situation in the same way as excessive time without connection. An extended duration without connection is given only by way of example, and the teaching in PhiScience is not limited to distance. While PhiScience does not have the discussion of how to trigger the alarm that is contained in the Patent and discussed by Mr Causey, it does teach the use of alarms in critical situations that can affect a system involving separate components and I agree with Mr Treneman that a skilled person would have understood a lack of proximity to be such a situation.
451. At one point it appeared that there was a dispute about whether Integers 42C (relating to a dispensing unit “free of user output components for providing the flow information from the local processor to a user”) and 45C (relating to a dispensing device “for attachment to a skin surface of a patient”) were disclosed by PhiScience. There was some skirmishing in the expert reports, and the submissions on these integers were somewhat like ships passing in that Mr Waugh QC considered them only as aspects of obviousness. Ultimately, Mr Tappin QC also noted that if they were not disclosed, they would be obvious, so I deal with them below as part of that analysis.
Conclusion on Novelty
452. On the evidence and in the light of the submissions, I find that all disputed aspects of Claim 2 were disclosed by PhiScience.
PhiScience obviousness/inventive step
453. Roche contends that all of the disputed integers are obvious when viewed in the light of the disclosure of PhiScience, even if they are not anticipated. Insulet contends that none of them is obvious in the light of PhiScience
454. I deal with the arguments on obviousness in respect of each integer in turn below. I consider first the differences between the prior art and the inventive concept of each claim as construed; and secondly, whether those differences involve steps which would have been obvious to the skilled person or whether they involve any degree of invention.
455. Mr Waugh QC drew my attention to the increased risks of hindsight in the context of inventions such as that in issue here. The authorities warn repeatedly of the danger of hindsight which may lead a court to treat as obvious a relatively simple step which gives rise to significant benefits, even though that step would not have been obvious to the skilled person at the relevant time. The leading authority on this topic, which has been repeatedly approved, is that of Moulton LJ in British Westinghouse Electric and Manufacturing Company Ltd v Braulik (1910) 27 RPC 209 at page 230:
“… I view with suspicion arguments to the effect that a new combination, bringing with it new and important consequences in the shape of practical machines, is not an invention, because, when it has once been established, it is easy to show how it might be arrived at by starting from something known, and taking a series of apparently easy steps. This ex post facto analysis of invention is unfair to the inventors, and, in my opinion, it is not countenanced by English patent law.”
General points on obviousness in PhiScience
456. Roche’s principal case on Claim 1 does not focus primarily on obviousness. Roche submits that once “directly connected with the body of the patient by means of a cannula” is correctly understood, as in its case on novelty, then it is obvious that this involved both tubelessness and attachment to the skin of the patient.
457. In the alternative, Roche submits that if those concepts are not directly disclosed, the skilled person would have regarded it as obvious to implement a tubeless design and to attach the fluid delivery device directly to the skin once the controls and display had been moved onto a remote control and the device made smaller and less obtrusive, avoiding the disadvantages of using connecting tubing.
458. Insulet submitted that if Roche’s case on disclosure of these integers failed on the construction point then obviousness based on PhiScience would not assist Roche.
459. PhiScience explains its purpose on page 3 of the Application: making the dispensing unit smaller by reducing the space required on the device for the operating elements so as to achieve “the smallest possible and unobtrusive construction of the pump together with unobtrusive operation”.
460. Insulet contends that the key teachings of PhiScience are focussed on the remote control with the interface controls removed from the pump and externalised to that remote control but otherwise involving a conventional pump and attachment mechanism for fluid delivery which is very different from the inventive concept of the Patent. Insulet says that the skilled person would not have been surprised by the teachings of PhiScience in the light of the CGK and that it does not suggest to the skilled person that there is a way of configuring a CSII pump to attach it to the body and do away with external tubing sets: the focus of the teaching is on the remote control.
461. Insulet also submitted that, as PhiScience required a remote control which formed part of another electronic device, PhiScience would not be regarded as a good starting point for further development by the skilled person and would have been put to one side. I have already held the factual premise for that submission to be incorrect.
462. The knowledge of the skilled person or team is discussed at paragraphs 24-28 above. It would include knowledge of existing products as well as knowledge of relevant written materials including, but not only, product literature. The initial approach of the skilled team to designing a new pump is discussed at paragraphs 73-79. I conclude that the skilled team would have been aware of PhiScience and would have considered it, along with other options, when approaching the design of a new insulin pump.
Applying Pozzoli
463. As explained by Kitchin LJ in Medimmune Ltd v Novartis Pharmaceuticals UK Ltd & Ors [2012] EWCA Civ 1234 at [93], the task for the Court is to approach the prior art with care as the skilled person would have done at the date of the prior art, in the light of the CGK and to “… evaluate all the relevant circumstances in order to answer a single and relatively simple question of fact.”
Inventive concept
464. Jacob LJ addressed identification of the inventive concept in Pozzoli at [17]-[18]:
“What now becomes stage (2), identifying the inventive concept, also needs some elaboration. As I pointed out in Unilever v Chefaro [1994] RPC 567 at page 580:
It is the inventive concept of the claim in question which must be considered, not some generalised concept to be derived from the specification as a whole. Different claims can, and generally will, have different inventive concepts. The first stage of identification of the concept is likely to be a question of construction: what does the claim mean? It might be thought there is no second stage - the concept is what the claim covers and that is that. But that is too wooden and not what courts, applying Windsurfing stage one, have done. It is too wooden because if one merely construes the claim one does not distinguish between portions which matter and portions which, although limitations on the ambit of the claim, do not. One is trying to identify the essence of the claim in this exercise.”
465. Insulet explains that the Patent’s overall purpose is to stake a claim over devices which are conceptually small, lightweight, low cost, simple to use, reliable, programmable, comfortable, capable of being adhesively attached to the patient’s skin and such that the device lends itself to being disposable in nature. It submits that this represents “a dramatic change in pump design”.
466. The device claimed under the Patent is described by Insulet as being a tubeless patch pump. Mr Causey’s written evidence stated that the integration between the TPAT and the EPA claimed in Integer 1H (and 2I, 3A, 42A and 45B) is one principal aspect of the inventive concept of the Patent as it means that the standard tubing set used with known pumps has been removed so that the device can be attached to the skin of the patient.
467. Mr Causey also stated that the removal of user components to a separate remote control claimed in Integers 1 D, E and G and Integer 42C is the other principal aspect of the inventive concept as it allows the reduction in size, weight and complexity of the device so that it may be attached to the skin of the patient and can be disposable.
468. Mr Causey also dealt with the specific inventive concept of other relevant Claims and I discuss these below as relevant.
469. Mr Treneman did not express a clear opinion on the inventive concept of the Patent or of specific claims. He understood the Patent to be proposing a low cost and disposable pump and identified the benefits of removing input and output components from the housing of the fluid delivery device as being of importance in the Patent.
470. During submissions, Mr Tappin QC focussed primarily on Integer 1G and identified the inventive concept as follows: “The inventive concept of the Patent is a remote-controlled ambulatory infusion pump (with a housing containing a wireless receiver, a processor, a reservoir, a dispenser and an exit port assembly which can connect to a transcutaneous delivery means) which has no electromechanical controls on the housing. It is the removal of all electromechanical controls from the housing which allows the device to be made smaller, lighter, less complex, cheaper, and hence disposable”. I did not understand Roche to contend that Integer 1H did not also form part of the inventive concept of the Patent.
471. I deal with the inventive concept of each claim as relevant below when assessing obviousness.
- Integer 1B: “an exit port assembly adapted to connect to a transcutaneous patient access tool”; Integer 1H: “wherein the transcutaneous patient access tool is integrated into the exit port assembly”
472. These integers are integral to the ‘tubeless’ solution and the implications of such a solution and are core to the inventive concept. I consider below whether it would be obvious to adopt a tubeless solution based on PhiScience if, contrary to my finding above, Insulet is correct on the construction of page 7 of PhiScience and it does not disclose the concepts claimed in Integers 1B and 1H.
473. The mechanism for attaching the fluid delivery device is crucial to the question of obviousness in respect of Integers 1B and 1H. The Patent does not claim ‘tubeless’ attachment as such (for reasons relating to patent drafting practice which were discussed during the hearing). However, if it is obvious in the light of PhiScience to move to a tubeless solution, whereby the pump is connected to the body of the patient, it follows that the EPA, TPAT and Integration are also obvious, notwithstanding the fact that some engineering effort will be required to make them functional, and that the teaching of Integers 1B and 1H will lack inventiveness. The key issue is whether tubelessness was an obvious next step from the teaching of PhiScience (on the alternative construction).
Evidence/submissions on ‘tubelessness’
474. Roche submits that it would have been obvious to move from the teaching of PhiScience to a tubeless outcome and that no degree of invention would have been required.
475. In summary, Roche contends that, once the controls and display had been moved to a remote control and away from the fluid delivery device, it would have been obvious to locate the device at the infusion site as this would avoid the known difficulties with using tubing. This contention was supported in Mr Treneman’s written evidence, albeit fairly briefly as much of his focus was on the disputed disclosure of the direct connection between the fluid delivery device and the body of the patient. When addressing obviousness, he contended that the skilled person would have viewed the problems of air bubbles in tubing and the inconvenience of tubing as considerable such that, once the teaching of PhiScience had been reviewed, it would have been obvious to take advantage of the lighter and smaller pump and to attach it directly at the infusion site.
476. Insulet contended that Mr Treneman’s evidence was fundamentally tainted by hindsight and did not represent the views and understanding of the skilled person at the priority date. Insulet submitted that the inventive concept which underpinned the move to a tubeless arrangement was a significant step from the CGK and from the teaching of PhiScience and would have involved significant inventive effort from the skilled person.
477. Insulet relied on the evidence of Mr Causey. He pointed out that problems arising from the use of tubing were not discussed in PhiScience. In his view, the industry was able to handle those problems using incremental improvements to tubing and this would have been the starting point for the skilled person. Mr Causey also gave evidence that a fundamental change in mindset would be required to think about a tubeless design and that implementing such a design would require multiple barriers to be overcome, requiring considerable ingenuity.
478. Insulet criticised Mr Treneman’s evidence as providing no substantiated reasons for the conclusion that moving to a tubeless pump would be obvious over PhiScience. During cross‑examination, Mr Treneman accepted that such a change would require a new design and that, as against existing products such as the MiniMed, that change would have been fundamental, particularly in the light of the conservative nature of those at MiniMed. He did not accept that this meant that such a step would be inventive for a skilled person who was not already working at an incumbent.
Assessment
479. The mindset of the skilled person at the priority date would have involved the use of external tubing and this would have been seen as a good basis for further action. The evidence of the experts has been helpful in assessing how willing the skilled person would have been to identify a different solution and regard it as obvious.
480. There is considerable tension between Mr Causey’s view of the likely approach of the skilled person (which was in my view coloured to some extent by his own experience as someone who was deeply involved at one of the principal incumbents at the relevant time) and that of Mr Treneman, who was not, but who might be seen as a proxy for a notional new entrant (although I consider there to have been a degree of hindsight in some of his views).
481. In weighing the evidence of the experts, coming from their different individual perspectives, I bear in mind that the position and mindset of the new entrant is as important as that of incumbents:
“Obviousness is tested against the mental and developmental norm of a notional uninventive person skilled in the art. In doing that the law is protecting not only established businesses which may wish to adopt new products, processes or designs or modify existing ones but also the new entrant who has employed persons skilled in the art to help him get into the market. Each of those categories of trader must be free to adopt what is obvious. Therefore it is legitimate to approach the prior art as if it had been collected and put on the desk of the new entrant at the priority date.” Brugger v Medicaid at page 653.
482. The experts agreed that the mindset of the skilled person, whether at an existing market participant or a new entrant, would have been conservative. The degree of conservatism would have varied, with new entrants more likely to move away from specific attributes of existing products and less likely to take quite such an incremental approach to design as companies with products on the market, who would have had to have regard to the preferences of existing patients, but the approach was generally incremental.
483. I prefer the evidence of Mr Causey on this point. Given the overall teaching of PhiScience and its focus, plus the state of the CGK and the mindset of the notional skilled person, I conclude that, in the absence of a specific disclosure of a tubeless attachment in PhiScience, it would not have been obvious to the skilled person that the teaching of PhiScience enabled a move to a tubeless pump design and a skilled team working on insulin pumps at the time would have required significant inventiveness to move from PhiScience to the claims in Integers 1B and 1H.
- Integer 1G: “wherein the housing is free of user input components for providing flow instructions to the local processor”
484. Roche contends that the removal of all the controls is disclosed by PhiScience or, in the alternative, that it would be obvious to remove those buttons achieving the same result as Integer 1G. It refers to the evidence of Mr Treneman who concludes that, while PhiScience has a preferred embodiment which includes emergency operating elements such as emergency off and emergency bolus buttons, this does not exclude the possibility of a device with no such buttons and that, given the overall objectives of PhiScience, a skilled person would see that removing all control elements would be an obvious route to achieve a small device.
485. Insulet refers to the specific approach in PhiScience itself as dissuading the skilled person from regarding the removal of all the buttons as obvious. It also relies on the concerns which it suggests the skilled person would have had about the overall PhiScience proposal which I have discussed and dismissed above.
486. During cross-examination, Mr Causey accepted that removal of all the buttons from the fluid delivery device would be an option. During that portion of his cross‑examination, he was considering a hypothetical situation in which the dispensing device was attached to the body of the patient. However, I do not regard that as crucial to an understanding of his evidence on the basic point, namely that to the skilled person an alternative to retaining some controls on the device would be to remove them all. Mr Causey made no reference to possible safety concerns arising from the removal of the buttons, perhaps in the light of the fact that he had already agreed that an alternative to safety buttons would be the removal of the device (emergency stop) or the use of an insulin pen (emergency bolus).
487. I conclude that on the balance of probabilities the skilled team would have regarded it as obvious to remove all buttons from the fluid dispensing device once the teaching of PhiScience about the removal of some controls had been understood. This would not have required any ingenuity or inventiveness.
- Integer 2M: “and further comprising a proximity alarm”
488. Roche contends that, even if such an alarm is not disclosed, it is obvious once the controls are separate from the fluid delivery device. Mr Treneman’s evidence supported this view.
489. Insulet submitted that this was not taught in PhiScience itself and that, in such circumstances, there would be no impetus for the skilled team to consider it.
490. Mr Causey’s written evidence suggested that:
“In general, if the skilled person was designing a system which included (a) an insulin pump carried or worn on the body which was free of user input component’s to provide flow instructions on its housing and (b) a separate remote control device which had user input components for providing the flow instructions to the fluid delivery device, that skilled person might consider adding a proximity alarm to reduce the risk of becoming separated from the remote control device.”
491. There was some dispute as to whether this evidence would hold good only where all controls were removed from the fluid delivery devices - i.e. where there would be a safety concern if the remote control and the fluid delivery device became separated. Mr Causey’s evidence seems to me to have been primarily concerned with the risk of separation or loss, rather than safety as such.
492. I conclude that, once it is taught that operating controls are removed from the fluid delivery device, the skilled person would find it obvious to consider adopting such an alarm, not least in circumstances where the concept of an alarm (albeit giving as an example separation for a period of time rather than over a distance) is already mentioned in the prior art.
- Integer 3A: “a kit including a system including a plurality of fluid delivery devices for delivering fluid to a patient”
493. Roche submitted that there is nothing inventive in providing the patient with a back‑up dispensing device in case the other was lost or stopped working and that an objection based only on cost would not prevent that from being obvious.
494. Insulet contended that as PhiScience is following the traditional model of an expensive, reusable device it would not be obvious to provide a kit containing more than one device.
495. The experts differed on the likely view of the skilled person. Mr Treneman considered that the skilled person would not necessarily have followed the industry approach to pumps once the controls were removed to a remote device. In those circumstances, he suggested that “a cheaper device, if it could be made sufficiently cheaply, would have the potential to be disposable” meaning that plural devices would be more likely. He also suggested that just because it would be expensive to provide a backup device would not make a decision to do so inventive.
496. Mr Causey explained that it would be unfeasibly expensive to provide a back‑up pump in the light of resistance to reimbursement on the part of the reimbursement agencies. During cross-examination, he also noted that the norm was for patients to carry a syringe as a back‑up device.
497. Given the mindset of the skilled person and the skilled person’s likely view at the time of the nature of pumps or fluid delivery devices, I conclude that, irrespective of cost and commercial considerations, it would not have been obvious to move from the teaching of PhiScience to disposable fluid delivery devices and then to the provision of a kit containing multiple devices. The skilled person had an obvious alternative solution to concerns about, for example, losing or misplacing the device, which would have been to carry a syringe. In the absence of any teaching in PhiScience directed towards disposability or the provision of multiple devices, it would require an inventive step to provide multiple devices.
498. To the extent that any dispute remains about Integer 3B “and a single remote control device separate from the fluid delivery devices”, this is resolved by the conclusion in respect of 3A as there are no “fluid delivery devices” in the kit, but only a single device.
- Integer 3M/3N: “wherein each fluid delivery device includes a bar code … and the remote control device includes a barcode scanner”
499. Roche submits that the use of a barcode / barcode reader system was an obvious means of ensuring pairing of the remote control and the dispensing device. PhiScience already teaches pairing and describes pairing the pumps and the remote control by means of a serial number. Roche noted that PhiScience teaches the importance of pairing to ensure secure communication between the remote control and the dispensing unit and relied on the evidence of Mr Treneman that, while one way of implementing a pairing solution would be for the user to enter the serial number of the pump into the remote control, barcoding was an alternative obvious means of doing so, which would have been safer and less prone to error.
500. During cross‑examination, Mr Treneman referred to his own use of barcodes in the pharmaceutical industry. However, Mr Treneman agreed with Mr Waugh QC that he had not previously referred to his previous experience in the pharmaceutical sector and had been unable to locate any documents which could assist in understanding the context.
501. Mr Treneman reiterated in cross-examination the view expressed in his written evidence that the use of barcodes was very common and well understood commercially and that once a need to pair was established it would have been an obvious alternative to the method disclosed by PhiScience. Mr Waugh QC and Mr Treneman did not agree on the precise teaching of PhiScience and whether it disclosed the provision of pre-encoded devices or merely devices in which the serial number could be used for pairing.
502. Insulet’s position was that PhiScience does not teach the use of barcodes to pair dispensing and operating units and that this concept had not been used to pair medical devices such as insulin pumps in September 2000 so that the use of barcodes in that context would not have occurred to the skilled person without invention.
503. Insulet suggested that Mr Treneman’s basis for suggesting that the use of barcodes would be obvious was clearly hindsight and without proper foundation and that Mr Treneman’s reliance on early (largely Japan-led) efforts at standardisation gave no reasons for suggesting that they would have been known to the skilled person at the priority date in the UK. There had been no suggestion in Mr Treneman’s written evidence that barcodes formed part of the CGK.
504. Insulet referred to the comments of Arnold J (as he then was) in Generics (UK) Ltd (t/a Mylan) v Warner-Lambert Company LLC [2015] EWHC 2548 (Pat) at [124]:
“Counsel for Warner-Lambert submitted that matter relied on as being common general knowledge must be shown to be common general knowledge in the UK, but counsel for Mylan and Actavis disputed that this was necessary. Although I only received limited argument on the point, it seems to me that, at minimum, it must be shown that the matter in question was common general knowledge in the UK. The reason for this is that, whether one is concerned with the validity of a European Patent (UK), or a UK patent, one is concerned with a right in respect of the UK. It is true that the prior art may have been published anywhere in the world, but I do not think that alters the need for the skilled team to consider that art as if they were located in the UK. I do not think it matters that a fact was common general knowledge in (say) China, if it was not common general knowledge here.”
505. Mr Causey gave evidence that PhiScience’s solution of pairing the remote control unit and the fluid delivery device using a serial number for doing so is a sensible way of arranging for pairing in a system where the two units would be paired for a considerable period of time so that there would be little downside to manual pairing and no motivation for the skilled person to look further.
506. During cross-examination, Mr Causey discussed with Mr Tappin QC his reasons for dismissing barcodes as an obvious option which would have been considered by the skilled person. He accepted that barcodes were known commercially, but noted that diabetic patients, the users of the system, would not have been familiar with barcode scanners at the time. He also confirmed in his written evidence that the cost of using barcodes would only be justified in circumstances where pairing was important.
507. I have concluded above that the use of barcodes did not form part of the CGK, whether in Japan or elsewhere. The evidence does not show that barcodes had been used in the medical devices field at the priority date. However, that does not mean that the use of barcodes would necessarily be inventive over the prior art.
508. The experts agree that barcodes were known commercially at the priority date and that they provided a quick and easy way to pair devices. Despite Mr Waugh QC’s submissions, neither expert suggested that barcodes were known only in Japan; Mr Causey’s comments about lack of knowledge related to the knowledge of patients, rather than of the skilled person and was narrowly focussed on users of insulin pumps. When pressed by Mr Tappin QC, Mr Causey confirmed that the main point in his written evidence was not that it was inventive to consider the use of barcodes as an alternative means of achieving pairing but rather that the skilled person would consider implementing barcodes only where pairing was important, as only then would the costs of doing so be justified.
509. Mr Treneman’s specific oral evidence about his previous use of barcodes in the pharmaceutical sector was not of particular probative value, but on several key points, including the importance of pairing and knowledge of barcodes more generally, his evidence was helpful and, in practice, there was little difference between the views of the experts on the possibility of using barcodes to pair devices.
510. The key point came down to whether it would have occurred to the skilled person to look beyond the method disclosed by PhiScience.
511. Mr Waugh QC submitted that it would not, because PhiScience taught a method of pre‑encoded devices. Mr Causey considered that the cost of barcodes would preclude their use unless pairing were important. Mr Tappin QC noted that Mr Causey had not raised the ‘pre-encoded point’ in his evidence and that, once the pairing was important, the skilled person would have considered alternatives and would not have been deterred by cost. Both Mr Causey and Mr Treneman ultimately agreed that PhiScience taught the importance of pairing.
512. Looking at this integer, and the claim in the round, I am not persuaded on the balance of the evidence that it would have required invention for the skilled person to consider the use of barcodes to achieve pairing, once it was taught that pairing was important. I consider that, having read page 10 of PhiScience, the skilled person would have concluded that secure pairing of the remote control and the dispensing device was important, particularly in the overall context of the disclosure of PhiScience. I do not accept that the skilled person would have understood the teaching of PhiScience as being so clear that there was no motivation to consider alternative possibilities; that being so, the use of barcodes would have been an obvious option to consider.
513. I conclude that Integer 3M/N is obvious over PhiScience.
- Integer 42C: “and wherein the housing is free of user output components for providing flow information from the local processor to a user”
514. Roche relies on the fact that PhiScience describes the division of elements between the dispensing unit and the operating unit. This portion of the description says that the operating unit is responsible for the operation, control and programming of the device, display and data input. “Display” is said to include matters such as doses administered, programming, control and other data. Roche submits that, as part of the overall teaching of PhiScience is to remove or reduce the need for the user to access the dispensing unit, this disclosure renders it obvious not to include any user output components on the dispensing device.
515. Insulet disagrees, and submits that the passages from the disclosure of PhiScience on which Roche relies say nothing about the absence of output components from the dispensing unit, noting that the closest reference is to a claim which mentions that the operating unit may include elements “for function or data display” (Claim 7). Insulet argues that, if the remote device is to be small and integrated into another device, it would be difficult to include all the display functionality on that device and concludes that, in the absence of clear teaching otherwise, the skilled person would have no reason or motivation to abandon the established approach, under which all dispensing units had displays of some form.
516. The expert evidence on the understanding of the skilled person tended towards the view that the removal of all elements of display to the remote control function would have been an obvious step in the context of the overall disclosure of PhiScience, with the dispensing unit being clear of all user output components.
517. Mr Treneman thought that PhiScience was disclosing a dispensing device with no display, or at the very least that a pump with no output components was obvious. Mr Causey’s written evidence was more tentative, stating that it was not clear that the dispensing unit described by PhiScience has no user output components, and suggesting that PhiScience contemplates some remaining on the dispensing unit. During cross‑examination, Mr Causey agreed that the removal of all of the user output controls would not have been inventive.
518. I conclude that the skilled person would have considered it obvious to remove all the user output components and the user input components from the fluid delivery device and to locate them on the remote control.
- Integer 45C: “for attachment to the skin surface of a patient”
519. Roche submits that, as a matter of construction, this requires only that the device be suitable for attachment to the skin. Roche contends that any portable dispensing device would have that property but that, in any event, if the device is “directly connected with the body of the patient by means of a cannula”, it must be suitable for attachment to the skin and that it would be obvious, in the light of such direct connection to attach the device to the skin.
520. Insulet contends that, for essentially similar reasons to Claim 1, it would not be obvious to modify the traditional belt- or bra-worn dispensing unit to adhere to the patient’s skin as a “patch pump” without external tubing and that Roche’s submission to the contrary (that it is “hard to envisage that any portable dispensing device could be unsuitable for [skin attachment]”) is pure hindsight.
521. Roche relied on the evidence of Mr Treneman to support its position. His view was that, if the device were located at the infusion site, it would be obvious to attach it to the patient’s skin, and that this could be achieved simply, for example by using adhesive tape.
522. Mr Causey disagreed, primarily owing to his view of the teaching of the patent in respect of Integer 1G. Owing to the shortness of time during the hearing, the passage of Mr Causey’s cross-examination which dealt with this issue was very abbreviated and, in hindsight, rather difficult to understand from the transcript but he agreed that, on the construction contended for by Roche, then it would have been obvious to attach the fluid delivery device to the patient’s skin.
523. I disagree with Roche that it would have been obvious to the skilled person at the priority date that any portable dispensing device would be suitable for attachment to the skin of the patient. There is much force in Insulet’s submission that this is pure hindsight. If the fluid dispensing device is of the type known in the prior art, it would not be obvious to attach such a device to the skin.
524. However, if PhiScience is construed as above in the context of novelty, the skilled person would have regarded it as obvious to attach the device to the skin of the patient.
Summary on obviousness over PhiScience
525. In summary, I conclude that the solutions taught in Integers 1G, 2M, 3M/N and 42C (and the equivalent teaching in other Claims and Integers) would have been obvious to the skilled person over PhiScience. If PhiScience discloses a tubeless attachment to the patient’s body, I conclude that Integers 1B/H and 45C (and the equivalent teaching in other Claims and Integers) would as a consequence also have been obvious. The consequence is that on that construction all Claims in Issue other than Claim 3 are obvious. On the alternative construction where PhiScience does not disclose a tubeless attachment, I conclude that Integers 1B/H and 45C would not have been obvious over PhiScience. Given the importance of the concepts in Integers 1B and 1H to the inventive concept of Claim 1 and of the Patent as a whole, I conclude that if the Patent is not anticipated by PhiScience it is also not obvious.
MiniMed - obviousness / inventive step
General points on disclosure
526. By the end of the trial, the only remaining dispute about the disclosure of MiniMed related to the nature of the outlet shown schematically in Figure 1 above (detail shown below):
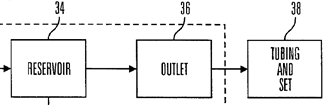
527. Roche submits that this shows an EPA adapted to connect to a TPAT as in Integer 1B (and the equivalent language in other integers of Claim 1 and in other claims). Insulet disagrees, largely by reference to the evidence of Mr Causey on the meaning of EPA and TPAT.
528. To recap, as set out at paragraphs 145-148 above, an EPA is a means of connecting the flow path from the reservoir to the flow path through the TPAT. As set out at paragraphs 149-150 above, the TPAT is an element that pierces the skin and enables the continuous transcutaneous infusion of insulin, such as a cannula or array of microneedles. Given that construction, I conclude that there is no basis to distinguish the disclosure of MiniMed from that of the Patent insofar as it discloses (i) an outlet which is a means of connecting the flow path from the reservoir (the EPA); and (ii) a means of enabling the continuous transcutaneous flow of insulin which pierces the skin (the TPAT). To this extent, there is no distinction between the disclosure of MiniMed and that of the Patent.
529. Roche accepts that MiniMed does not disclose a device wherein the TPAT is integrated into the EPA as claimed by the Patent.
The Pozzoli analysis
Inventive concept
530. I have dealt with the inventive concept of the Patent above in the context of PhiScience.
531. MiniMed seeks to improve comfort and discretion for patients by the removal of functions to a remote control; by improving the programming of insulin delivery and by resolving some of the difficulties arising from the use of tubed devices.
532. The obviousness attack in respect of each individual integer is considered separately below. There is significant overlap between some aspects of the obviousness attack based on MiniMed and that based on PhiScience. To avoid unnecessary repetition, the points made above in respect of PhiScience are where relevant referred to below.
533. Overall, Roche submits that two of the key objectives of MiniMed are: to provide an infusion device that can be used and controlled discreetly; and to deal with known problems arising from the use of external tubing. Roche contends that MiniMed’s approach to the first objective, the removal of controls to a remote controller, would be perceived by the skilled person to lead to a device that would have no controls on the infusion device. The obvious consequence of that, it is submitted, is that the infusion device could be directly attached at the infusion site without the use of tubing. If correct, this would have the consequence that the two primary aspects of inventiveness in the Patent contended for by Insulet would be obvious over MiniMed. I therefore deal first with the claims that go to these two issues.
- Integer 1G: “wherein the housing is free of user input components for providing flow instructions to the local processor” / Integer 42C: “and wherein the housing is free of user output components for providing flow information from the local processor to a user”
534. Roche notes that MiniMed teaches that the infusion device could be made more discreet and more easily controlled (and therefore smaller and lighter) by removing controls from the device itself and contends that, in the light of that teaching, it would not be inventive to remove all of the controls from the infusion device. MiniMed discloses an embodiment which has no user input or output components on the device. In such an embodiment, where only a remote control would be used to programme and control the fluid delivery device, Roche submits that the skilled person would recognise that a more complex remote control, including a keypad and screen would be obvious.
535. Much of the evidence on this issue has already been discussed in the context of PhiScience.
536. In his written evidence on MiniMed, Mr Causey suggested further reasons why the skilled person would not have regarded it as obvious to put all of the controls on the remote control device in the light of MiniMed. Briefly, these were that the main embodiment in MiniMed is a simple remote control which is additional to the functionality of the pump and that the overall teaching of MiniMed clearly points towards the controls remaining on the pump. His written evidence stated that the skilled person would not consider it obvious to develop a pump without controls. This appears largely to have been based on his views of the preferences of patients and the conservatism of those involved in pump design. However, in both his written evidence and during cross‑examination, Mr Causey acknowledged the alternative embodiment in MiniMed which envisaged moving all user outputs and inputs to a separate device. During cross‑examination, Mr Causey agreed that the skilled person would recognise that, if that embodiment were adopted, it would be appropriate to use a more complex keypad arrangement and a display screen on the remote control. He did not agree that merely identifying the possibility of a set up with all the controls on the remote control would lead the skilled person to take the additional step of moving to a body adhered device, and could see no motivation for the skilled person to remove all the controls without a useful reason for doing so.
537. Mr Treneman disagreed with these specific considerations and did not regard them as likely to deter a skilled team from concluding that the control functions could be moved entirely from the fluid delivery device. His principal points were primarily those already mentioned in respect of PhiScience.
538. Having considered the evidence of the experts as to the likely approach of the skilled team in the light of the specific teaching of MiniMed and the CGK, I conclude that it would not have involved an inventive step to proceed from the teaching of MiniMed to a device with no user input or output functions (including a display) on the fluid delivery device.
- Integer 1B: “an exit port assembly adapted to connect to a transcutaneous patient access tool”; Integer 1H: “wherein the transcutaneous patient access tool is integrated into the exit port assembly”
539. Roche submitted that, if it were obvious to move to a fluid delivery device devoid of control functions, it would also be obvious to remove the tubing, so resolving the problems with tubing identified in MiniMed, and subsequently obvious also to proceed to a device for attachment to the body of the patient. In such a configuration, there would be a direct connection between the EPA and the TPAT. In Roche’s submission, this rendered the key inventions of the Patent obvious over MiniMed.
540. Roche relied on the evidence of Mr Treneman. His evidence on obviousness in the context of MiniMed was a little fuller than in the context of PhiScience (where his principal focus was on the disclosure of a direct connection between the fluid delivery device and the body of the patient). In essence, however, his view was that, once the controls and display had been moved to the remote control, there would be no need to access the device which could be hidden away beneath the patient’s clothing. That would lead the skilled person to consider doing away with the tubing and all the related problems by locating the device at the infusion site, and to attach the cannula used to deliver Insulin directly to the outlet of the device.
541. During cross‑examination, Mr Treneman acknowledged that taking such steps would have required a significant and fundamental re-design (particularly in the context of prior art such as MiniMed) given the CGK and the conservative mindset of those at MiniMed. In this portion of his cross‑examination, he sought to distinguish the conservatism of those at MiniMed from that of the skilled team. I accept that on some issues those at MiniMed might have been more conservative than the notional skilled person or team, particularly when modifying existing products but, as mentioned above, both experts accepted that the mindset of the skilled team designing an ambulatory insulin pump in 2000 was conservative.
542. Mr Causey disagreed fundamentally with Mr Treneman’s view of the obvious steps based on MiniMed. In addition to the points he made in the context of PhiScience, he also referred to the specific teaching of MiniMed as being towards an expensive, durable infusion pump, explicitly intended to be connected using tubing and an infusion set and providing its own means of addressing the problems caused by tubing which it had identified.
543. In the light of the expert evidence, Mr Waugh QC submitted that there was no basis to regard a move away from the use of tubing to a tubeless design with direct attachment to the body of the patient as obvious over MiniMed. Mr Waugh QC pointed to a tubed design as being fundamental to the teaching and disclosure of MiniMed, noting that the choice to use tubing is a significant feature of the solution taught by MiniMed to two drawbacks of the traditional CSII pump:
· first: “the inability to conceal an external infusion pump and catheter tubing from view” is solved by the remote control, allowing the patient to operate a concealed device; and
· second: the need to prime the pump “to remove gas bubbles in the reservoir and/or tubing”, which requires shaking and expelling the bubbles resulting in loss of the medicine is solved by using a vibration device “to generate sufficient vibration to assist in removing gas bubbles from the fluid in the reservoir during priming” and thereby to remove air bubbles in the tubing.
544. In the light of the specific approach of MiniMed, Insulet submits that the skilled person would be unlikely to move to a solution based on the removal of all tubing.
545. Having considered the evidence of the experts as to the likely approach of the skilled team in the light of the specific teaching of MiniMed and the CGK and having considered the teaching in MiniMed specifically, I conclude that it would have involved significant inventive capacity to proceed from the teaching of MiniMed to the claims of the patent and that to do so would not have been obvious. The reasoning on obviousness in the context of PhiScience applies also to MiniMed, but with greater force.
546. As far as the secondary claims are concerned, much of the expert evidence on obviousness was the same as in the context of PhiScience:
· Integer 1I: “and wherein the reservoir is contained in the housing and has a volume in the range of 2 to 3 ml”: it is common ground that a reservoir volume of 2 to 3 ml was standard. MiniMed does not disclose the volume of the reservoir referred to but it is accepted by both experts that 3ml would be an obvious volume to use;
· Integer 2M: “and further comprising a proximity alarm”: MiniMed discloses various audible and vibration alarms. These are taught in specific contexts, and do not include an alarm which alerts the user in the event of excessive distance between the fluid delivery device and the remote control. Insulet contended that, as MiniMed assumes that the remote controller will replicate functions on the fluid delivery device, rather than replace them, a proximity alarm would not be an obvious step. Insulet also suggested that MiniMed implicitly teaches against such an alarm. Insulet relied on the evidence of Mr Causey in relation to PhiScience as equally applicable to MiniMed. I do not agree with Insulet’s contentions on the specific teachings of MiniMed. A proximity alarm would have been an obvious development, as discussed above;
· Integer 3A: “a kit including a system including a plurality of fluid delivery devices for delivering fluid to a patient”: no plurality of pumps is disclosed or discussed. It would not have been obvious to provide a plurality of pumps in the light of the teaching of MiniMed, for reasons similar to those already discussed above in the context of PhiScience;
· Integer 3M/3N: “wherein each fluid delivery device includes a bar code and the remote control device includes a barcode scanner”: Insulet refers to the express teaching in MiniMed as to the way in which the remote control and the fluid delivery device are to be paired and submits that the idea of using a bar code would not have been obvious to the skilled person. For the reasons similar to those in respect of PhiScience above, I conclude that it would have been obvious to the skilled person to move from the teaching of MiniMed to a bar code system;
· Integer 42C: “and wherein the housing is free of user output components for providing the flow information from the local process to a user”: this integer has been discussed above;
· Integer 45C: “for attachment to a skin surface of a patient”: the evidence and submissions on this integer have already been discussed in the context of Integers 1B and 1H above. I conclude that it would not be obvious to proceed from the teaching of MiniMed to a fluid delivery device adhered directly to the skin of a patient.
Summary on obviousness over MiniMed
547. In summary, I conclude that the solutions taught in Integers 1G, 1I, 2M, 3M/N and 42C (and the equivalent teaching in other Claims and Integers) would have been obvious to the skilled person over MiniMed. I conclude that Integers 1B/H, 3A/B and 45C (and the equivalent teaching in other Claims and Integers) would not have been obvious to the skilled person over MiniMed. My overall conclusion in respect of MiniMed is therefore the same as it would have been in respect of PhiScience had I not concluded that PhiScience discloses a tubeless attachment to the patient’s body: Claims 1 and 45C are not obvious over MiniMed. Given the importance of the concepts in Integers 1B/H to the inventive concept of Claim 1 and of the Patent as a whole, I conclude that the Patent is not obvious over MiniMed.
Added matter
548. The allegations of added matter fall into two categories: one arising from amendments made during prosecution of the application for the Patent; and one arising from the proposed amendments.
Legal principles
549. Added matter is to be identified using the simple test stated by Jacob J (as he then was) in 1995 and repeatedly cited by others: “I think the test of added matter is whether a skilled man would, upon looking at the amended specification, learn anything about the invention which he could not learn from the unamended specification.” Richardson-Vicks Inc’s Patent [1995] RPC 568 at [576].
550. As recently restated by Meade J in Philip Morris Products, SA & Anor v RAI Strategic Holdings, Inc & Anor [2021] EWHC 537 (Pat) at [112]-[120], the overall approach involves asking whether the claim as amended presents the skilled person with information about the invention which is not derivable directly and unambiguously from the original disclosure of the application as filed. As this is a question relating to the disclosure of the application as filed and the patent as granted/amended, it is a matter for the court, not the expert witnesses, although the court will carry out this task through the eyes of the skilled person.
551. Meade J’s Judgment referred to and quoted the three-stage test in Bonzel v Intervention Ltd [No 3] [1991] RPC 553 at [574]. This requires the Court:
(i) to ascertain through the eyes of the skilled addressee what is disclosed, both explicitly and implicitly, in the application;
(ii) to do the same in respect of the patent as granted (or as in this instance also as proposed to be amended); and
(iii) to compare the two disclosures and decide whether any subject matter relevant to the invention has been added whether by deletion or addition, bearing in mind that the comparison is a strict one in the sense that subject matter will be added unless such matter is clearly and unambiguously disclosed in the application either explicitly or implicitly.
552. As explained in Vector Corporation v Glatt Air Techniques Inc [2007] EWCA Civ 805 at [5] and [6], the policy rationale underpinning the rule against adding matter is to prevent the patentee from obtaining a different monopoly to that which the application originally justified. Third parties should be able to look at the application and understand the basis for the claimed monopoly. Meade J explained that the test for added matter is therefore “rooted in determining the disclosure of the application as filed and the patent as granted (or as proposed to be amended)” (Philip Morris at [118]).
The allegations of added matter
Added matter during prosecution
553. It is for Roche to establish that matter has been added. Roche’s added matter case on the prosecution of the Patent relates to the addition of Integer 1H and various disclaimers in the description in the Patent that were absent from the application.
554. Integer 1H reads “and wherein the transcutaneous patient access tool is integrated into the exit port assembly”. Claim 1 in the Application stated only that the claim covered a device comprising “an exit port assembly adapted to connect to a transcutaneous patient access tool”. How the EPA and the TPAT were to be connected was not specified and there was no mention of integration in the draft claims and, as in the Patent, there is no definition of EPA or TPAT.
555. Roche contends that the Application taught the TPAT as encompassing at least a standard infusion set which could attach to the EPA using a conventional Luer connector. It refers to various descriptive paragraphs:
· paragraph (70) which explained that the “fluid delivery device 10 of Fig. 4 also includes a Luer connector 71 for attaching a standard transcutaneous fluid delivery set to the exit port assembly 70”;
· Figure 9a which showed a standard infusion set, and paragraph (84) stated in relation to that figure that “infusion set 400 can be attached to fluid delivery device 10 by connecting the infusion set Luer connector 401 to the Luer connector 71 of the exit port assembly 70 of the device 10”;
· paragraph (101) which referred to “accessories such as an attachable transcutaneous infusion set, such as that described hereinabove”; and
· Claim 14 of the Application (which was dependent on Claim 1) included a Luer connector on the exit port assembly.
556. Roche states that this makes clear that references in the Application to a TPAT included the tubing of a standard infusion set, since this tubing could be connected (typically via a Luer connector) to the EPA according to Claim 1 and the teaching identified above.
557. Roche states that only paragraph (107) of the Application mentions ‘integration’. That paragraph reads:
“Fig. 13c shows a sterile infusion set assembly 407 including the transcutaneous infusion set 400 described hereinabove packaged in an infusion set pouch 406. The infusion set 400 includes an infusion set Luer 401 connected to infusion set flexible tubing 404 and terminating in an infusion set penetrating cannula 405. An optional set of infusion set wings 403 can be included to attach the infusion set 400 to the patient’s skin. In the preferred embodiment of fluid delivery device 100, the transcutaneous delivery means are integrated into exit port assembly 70, however in an alternative embodiment, the exit port assembly can be attached to infusion set 400. In this particular embodiment, it may be desirable to kit sterile infusion set assemblies 407 with any quantity of one of more of the sterile assembly packs 350, the fluid delivery device 10, the remote control device 100 or the therapeutic fluid supply 250.” ( Emphasis added by Roche)
558. Roche explains that this paragraph disclosed two options: either the integration of the transcutaneous delivery means into the EPA; or the attachment of the transcutaneous delivery means to the EPA, and that as the Application envisaged the use of a standard infusion set as a transcutaneous delivery means, no distinction is being drawn in this paragraph between ‘tubed’ and ‘tubeless’. Roche says that the plain meaning of the paragraph is to contrast ‘integrated’ delivery means with ‘attached’ delivery means - whether or not the delivery means included tubing.
559. Roche notes that, as well as adding Integer 1H (“and wherein the transcutaneous patient access tool is integrated into the exit port assembly”), the paragraphs and descriptions mentioned above have also been amended as between the Patent and the Application: the possibility of using a standard infusion set and Luer connector discussed in paragraph (70) of the Application is now stated by the Patent to be contrary to the claimed invention (at the end of paragraph [73]); the same possibility of using a standard infusion set and Luer connector shown in Figure 9a and discussed in paragraph (84) of the Application is now said at the end of paragraph [0087] of the Patent to be contrary to the claimed invention; and the reference in paragraph (101) of the Application is now said in the Patent to be contrary to the claimed invention.
560. In Roche’s submission, Insulet’s approach seeks to put a wholly different construction on this integer, and on the Patent as a whole, by seeking to exclude tubing from the definition of the TPAT, something that was not disclosed by the Application. Insulet’s position is that Integer 1H refers to a tubeless, but detachable connection to the EPA, rather than a permanent connection. Roche notes that this reflects the views of Insulet’s expert on how a skilled person would read the Patent (which were set out above at paragraph 201).
561. In summary, Roche contends that the Application does not disclose a device involving a tubeless (but detachable) connection of the TPAT to the EPA and that, as a consequence, either Integer 1H (and the other related integers) must refer to a permanent connection of the TPAT to the EPA, as in paragraph (107) of the Application, or there is added matter as the Application does not disclose a device which falls within Insulet’s construction of Integer 1H.
562. Insulet refers to various aspects of the Application which it says unambiguously disclose that the TPAT can be a cannula which is integrated into the EPA (as opposed to a standard infusion device that connects to it). During oral submissions, Mr Waugh QC submitted that two clear alternatives were disclosed in the Application:
(a) a standard infusion system (including tubing) adapted to connect to the EPA; and
(b) a TPAT which was integrated into the EPA.
563. Insulet’s position was that the reference in the Application to an integrated transcutaneous delivery means (option (b) above) was clearly to a cannula rather than a standard infusion system and that during prosecution the applicant disclaimed option (a) while maintaining option (b) in the Patent. In Insulet’s submission maintaining option (b) alone could not add matter as the skilled person would learn nothing about option (b) from the granted patent, which was not clear from the Application as filed.
564. Insulet referred to the disclosure in the Application to support its contention that a skilled person would have been in no doubt on reading the Application that option (b) (a cannula integrated into the EPA) was disclosed (all emphasis below added by Insulet):
· “[0016] According to a further aspect, the exit port assembly includes a tubular member for transcutaneously entering a patient. …” [A/3/41].
· “[0018] The present invention further provides another device for delivering fluid to a patient, including an exit port assembly adapted to connect to a transcutaneous patient access tool, … A housing contains the exit port assembly …” [A/3/42].
· “[0052] When the local processor 50 activates the dispenser 40, a specific amount of fluid exits the fluid delivery device 10 via the exit port assembly 70. The exit port assembly 70 can include elements to transcutaneously enter the patient, such as a needle or soft cannula, or can be adapted to connect to a standard infusion device that includes transcutaneous delivery means.” [A/3/46].
· “[0107] In the preferred embodiment of fluid delivery device 100, the transcutaneous delivery means are integrated into exit port assembly 70 …” [A/3/63].
565. Insulet also relied on the evidence of Mr Causey as to the understanding of the skilled person that the integration of the TPAT into the EPA was clearly disclosed. Insulet noted that Mr Causey relied for that conclusion on passages which were present in both the Application and the Patent itself (essentially paragraphs [0021] and [0055] of the Patent which are the same as paragraphs (18) and (52) of the Application).
566. A significant difficulty with both the Application and the Patent is that several phrases or acronyms which are key to understanding the claims and the invention are not explained, so that the skilled person (and ultimately the Court) must infer their meanings. I have already referred above to this difficulty in relation to ‘Exit Port Assembly’; ‘Transcutaneous Patient Access Tool’; and ‘Integrated’ when construing the Patent as granted. There were significant differences of opinion between the experts as to the meanings of these phrases in the Patent - see the discussion of EPA at paragraphs 145-148 above; of TPAT at paragraphs 149-150 above; and of ‘integrated’ at paragraphs 198-213 above. The understanding of these concepts and how they affect the scope of the monopoly claimed by Insulet is at the core of this added matter examination.
567. When discussing the Patent as granted, Insulet’s description of the inventive core, or key inventive concept, is of a ‘tubeless’ patch pump. This is closely connected to the meaning to be attributed to Integer 1H of the Patent and, in particular, the meaning of Integer 1H in combination with Integer 1B.
568. Mr Causey’s expert evidence considered the teaching of the terms ‘TPAT’ and ‘integrated’ as disclosed in the Patent. He explained that a skilled person would consider a TPAT to be the element that enables continuous transcutaneous infusion of insulin and that “… the TPAT is connected with and integrated into the exit port assembly. The two components will therefore connect directly to each other when the device is in use without any intermediate components … In practical terms, this means there is no tubing between the exit port assembly and the infusion site TPAT.”
569. He continued, at paragraph 123 of his first report:
“This “tubeless” design can be distinguished from existing infusion pumps in which there is exterior tubing between the pump and the infusion site. These small lightweight tubeless infusion pumps which are adhered to the body are sometimes known today as “patch pumps”. This arrangement enables the pump and the adhesive around the infusion site to provide protection for the infusion site from physical factors and infection. The exit port assembly and direct integration with the TPAT, without any external tubing, is quite different to the arrangement for the delivery of insulin in the continuous infusion pumps which were in use in September 2000.”
570. This theme was picked up in Insulet’s written submissions. Mr Waugh QC’s skeleton argument stated:
“A key feature of the invention is the “exit port assembly” which is connected to, and integrates, the “transcutaneous patient access tool” (“TPAT”) (e.g. a cannula that is inserted into the patient’s skin, or a microneedle array). This is a fundamental design change to previous tubed insulin pumps, which, as noted above, required users to attach the end of the tubing distant from the infusion set to the reservoir. In contrast, the TPAT of the device claimed in claim 1 is “integrated into the exit port assembly”, which in turn is contained within the housing of the device: Causey 1 ¶¶120–122.”
571. Insulet submitted that it made sense to read Integers 1B and 1H together because each informs the other. Mr Causey also made this point during cross‑examination, when he explained:
“A. … the definition of an "exit port assembly" is that it is an attribute that connects to a needle or a cannula, is integrated with the TPA[T].
Q. Okay, so that comes from the fact that it has to be integrated with the TPAT, not from it being an exit port assembly itself?
A. Again, the definition of the "exit port assembly" in the patent is a composite of all of the attributes that go with it. So you cannot take the exit port assembly phrase and try to parse that separately as some kind of a separate kind of attribute. It is in the context of all those things that connect to it.”
and Mr Waugh QC submitted:
“This “tubeless” design gave birth to an entirely new category of infusion pumps which became known as “patch pumps”. Instead of using tubing, these lightweight pumps adhered directly to the body, something that was enabled by the exit port assembly and TPAT being integrated into the housing. This was quite different to previous approaches at the priority date: Causey 1 ¶123.”
572. Insulet discussed the inventive core of the Patent as it would be understood by the skilled person in the context of infringement, referring again to the evidence of Mr Causey and relying particularly on his first report:
“It is clear from the features of the claims read in context of the overall disclosure in EP 764 that the fluid delivery device is designed to be ambulatory and attached to the skin and used for the continuous infusion of insulin. The overall concept of the system described in EP 764 can be summarised as a system of small, light-weight, low-cost, tubeless, disposable pumps which are managed with a separate remote-control device. EP 764 teaches that such a fluid delivery device can be achieved through two new inter-related and synergistic design features:
(A) the connection/integration of the TPAT and the exit port assembly means that the standard tubing set used with known insulin pumps has been removed and the device can be attached directly to the skin of the patient. The exit port assembly structure would also be new to the skilled person. Indeed, the entire class of device represented by EP 764 - the 'patch pump' - was new.
(B) the location of bulky, costly and complex user input components for providing flow instructions and confirmation of correct dose programming in a separate remote control reduces the size, weight, complexity and cost of the fluid delivery device which enables the patient to attach the fluid delivery device to their body and control their insulin treatment discreetly and more conveniently with the remote control, without accessing the fluid delivery device. The location of components in the remote control also makes the fluid delivery device itself considerably less expensive, allowing it to be disposable, and tubeless and slimmer and less likely to catch on clothing and jewellery.
I have been asked to relate the inventive concept of EP 764 to the specific features of the claims. The tubeless part of the invention is recognised mainly in features B, C and H which require the transcutaneous patient access tool to be connected and integrated within the exit port assembly (which is within the housing) to enable insulin to flow from the reservoir through the exit port assembly and transcutaneous patient access tool and into the user.”
573. Insulet subsequently explains that the purpose of Integer 1H is to “distinguish the claimed invention from previous devices with an external tubing and infusion set”.
574. Insulet’s principal response to Roche’s added matter contentions was that the Application disclosed that the TPAT can be a cannula integrated into the exit port assembly. Mr Riordan, who made the oral submissions on behalf of Insulet on this point submitted that this was a clear and distinct option and that the removal of another clear and distinct option cannot therefore add matter.
575. The key to the assessment of this issue lies in the third stage of the three stage test in Bonzel v Intervention Ltd, recently cited by Meade J in Philip Morris. At that stage, the task for the Court is to compare the disclosure in the Patent and that in the Application:
“… and decide whether any subject matter relevant to the invention has been added whether by deletion or addition, bearing in mind that the comparison is a strict one in the sense that subject matter will be added unless such matter is clearly and unambiguously disclosed in the application either explicitly or implicitly.”
576. The core question is therefore whether the addition of Integer 1H and the inclusion of the various disclaimers in the Patent changed the disclosure so as to tell the skilled person something about the invention which he could not learn from the Application. I conclude that it does.
577. I agree with Mr Riordan and with the other submissions by Insulet that the Application tells the skilled reader something about the potential use both of standard infusion sets and of other potential means of delivering fluid transcutaneously. However, I do not accept that the Application clearly and unambiguously excluded standard infusion sets from the meaning of ‘transcutaneous patient access tool’ as used in Claim 1.
578. I also agree that the Application tells the skilled reader about elements that could be included in the EPA, but I do not accept that the Application discloses that such elements could be integrated in the sense of Integer 1H as construed above.
579. The language in the description is far from clear but I consider that for a skilled reader, in the light of the CGK at the time, paragraphs 70, 9a and 101 would have been read as indicating that ‘a transcutaneous patient access tool’ as referred to in Claim 1 would include a standard infusion set.
580. The paragraphs referred to by Insulet do not disclose that a TPAT excludes a standard infusion set, nor do they disclose that a TPAT is integrated with the EPA in the sense of being attached directly but detachable as in Integer 1H.
581. The figure on the title page of the Application (which equates to Figure 1 of the Patent) does not clearly indicate what is or is not a TPAT and tells us nothing about whether it is fixed or detachable. Mr Causey stated in cross-examination that he would not infer that the protrusion shown in Figure 1 was a TPAT.
582. Paragraphs (16) and (52) describe transcutaneous elements that may be included in the EPA, but do not exclude a standard infusion set from the concept of TPAT as in Claim 1, nor do they disclose that elements which are ‘included in the EPA’ are detachable, or not fixed to the EPA.
583. Paragraph (18) reflects the language in Claim 1 of the Application, now Integer 1B in the Patent. It does not clarify the meaning of TPAT nor does it say anything about integration.
584. This leaves paragraph (107) of the Application. The question is whether this clearly and unambiguously discloses one option in which the transcutaneous delivery means was tubeless and integrated and one in which the transcutaneous delivery means was tubed and attached. In my view it does not: to reach that reading, particularly in the light of the CGK, requires the skilled person to make a significant leap and to infer that in paragraph (107) the phrase “In the preferred embodiment of fluid delivery device 100, the transcutaneous delivery means are integrated into exit port assembly…” excludes tubed transcutaneous delivery means. I conclude that it is much more likely that the skilled person, with a mindset focussed on tubed solutions, as we understand from the evidence of Mr Causey (with which Mr Treneman did not disagree), would have read paragraph (107) as distinguishing between integrated or fixed embodiments and attached or removable embodiments.
585. I conclude that, in order to arrive at the inventive concept now said to be disclosed by the Patent, the addition of Integer 1H was important and that it was equally important for the Applicant to disclaim the tubed embodiments which were clearly incorporated in the Application so as to teach the skilled person to think about something wholly different (in the words of Insulet) from what had been known in the past and to learn from the amended specification and the Patent that the inventive core of the Patent was tubeless and directly connected to the body of the patient in a way that was not fixed but detachable. That teaching is not clear and unambiguous in the Application.
586. Mr Riordan submitted that:
“We do not need to rely on the statements that various embodiments are contrary to the invention. They provide further reinforcement and support for our position on construction of claims, but we certainly do not need to rely on there being any length of tubing in between the exit port assembly and the transcutaneous patient access tool for the reasons my learned leader has already explained.”
587. I disagree and consider that the addition of Integer 1H to Claim 1 (and the related claims) plus the disclaimers in the specification would provide the skilled person with information about the invention which is not derivable directly and unambiguously from the original disclosure of the Application as filed and therefore add matter. The skilled person reading the Patent after amendment during prosecution would learn something new and different not only about the specific teaching of Claim 1 but also about the inventive core of the invention. As the other Claims in Issue either contain a feature equivalent to Integer 1H or are dependent on Claim 1, the consequence is that all the Claims in Issue are invalid by reason of added matter.
Added matter in the proposed amendments
588. Of the asserted claims, the added matter objection relates to proposed amended Claim 43. As this judgment is already long, I will focus only on that claim.
589. Roche explains its attack as follows:
· Claim 43 is (because of its dependency on Claim 42) to a device, system or kit according to Claim 1, 2 or 3 which also has Integers 42B, 42C and 43B. A system according to Claim 2 has a proximity alarm (Integer 2M), while a kit according to Claim 3 has fluid delivery devices including a bar code (Integer 3M) and a remote control device including a barcode scanner (Integer 3N);
· Roche contends that the Application does not clearly and unambiguously disclose a system with a proximity alarm which also has Integers 42B, 42C and 43B. While Claim 44 has a proximity alarm, it is dependent on Claims 1 and 43 and those claims do not include Integers 42B, 42C and 43B;
· Roche makes a similar point that the Application does not sufficiently clearly disclose a kit with fluid delivery devices including a bar code, and a remote control device including a barcode scanner, which also has Integers 42B, 42C and 43B. Claim 47, which has those features, is dependent on Claims 1, 43, 45 and 46 and those claims do not include Integers 42B, 42C and 43B. In addition, because of their dependency on Claim 45, those claims also require the kit to comprise a subcutaneous patient access tool for connection to the exit port assembly of the fluid delivery device;
· Roche’s essential argument is that none of the claims or descriptions of the Application has the combination of features now sought to be claimed. It refers to the authorities (most recently Conversant Wireless Licensing SARL v Huawei Technologies Co Ltd [2020] EWCA Civ 1292) and notes that the test for added matter is a strict one requiring that the proposed claimed subject matter is clearly and unambiguously disclosed in the Application. Ultimately Roche’s position is that, as Insulet has not identified anything in the claims or the application which discloses a system or kit with the features (and only the features) of the system or kit of proposed Claim 43, the amendments add matter.
590. Insulet responds by describing Roche’s contentions as ‘arid and technical’ on the basis that, while the specific claims in their original form referred to a ‘device’ only, the Application makes clear that the claimed devices may each be packaged as a system and a kit. Insulet also notes that Amended Claim 2 is original Claim 38 (dependent on original Claim 1) rewritten in long-hand form. It does not add anything to the granted claims, while amended Claim 3 is original Claim 40 (dependent on original Claim 1) written out in long-hand form.
591. The proposed amendments have been reviewed by the Comptroller of Patents, whose views were received just before trial. Most pertinently, the Comptroller observed that:
“The description as filed discussed the use of systems and kits interchangeably with a number of different feature configurations and it is felt no matter is added in amending the dependent claims to reflect this”.
592. Stepping back to consider the amendments which have been proposed as against the disclosure in the original specification, the Application as filed discloses kits and systems with the relevant features and the specification envisages a number of components in such kits and systems. In view of that, and acknowledging that the test is strict, when I consider Jacob J’s ‘simple test’, I conclude that the skilled person would learn nothing new from the proposed amendments which would not have been known from the original specification and that the third party would understand the basis for the claimed monopoly on that basis. The proposed amendments do not add matter.
Amendment
593. The Patent may be amended in line with the unconditional amendments submitted.